Traditionally, the doctor-patient relationship was somewhat directive. Patients would more often assume a relatively passive role, relying on the doctor’s recommendations without contributing much to the decision-making process. Today, providers still drive decision-making in some disease areas, but overall, we are much more likely to see situations that are characterized by shared decision-making between patient and provider.
Patients are typically more educated, informed, and engaged. In many cases, decision-making is no longer a “one-way street,” as physicians and patients are now more likely to work together to select diagnostic tests, treatments, and care plans based on a range of factors that can go beyond just clinical evidence. Increasingly, physicians and patients are jointly exploring the potential risks and rewards of any given treatment plan, as well as incorporating the patient’s perspectives, preferences, and values into the equation.
The concept of patient centricity is one that gets a lot of attention these days, but it’s not just relevant to the doctor-patient relationship. As patients become more educated about their diseases and treatment options, their expectations of those who develop and manufacture such treatments are higher. This creates opportunities for all key players in the healthcare ecosystem – including biopharma companies – to create better experiences and outcomes for patients.
For biopharma companies to take advantage of this opportunity, they need to weave the patient-centric mindset into the fabric of their organizations and across the patient journey. This impacts clinical research, education, diagnosis, treatment options, patient monitoring and all the functions of a biopharma company. The Medical Affairs (MA) organization is a key player in this effort, and our goal is to provide some guidance for MA decision makers to help ensure their success. In this article, we do the following:
- Define “patient centricity”
- Describe how patient expectations have changed and why these changes are important to biopharma companies
- Provide some concrete examples of how some MA teams in biopharma companies are being more patient-centric in today’s environment, hopefully sparking ideas regarding how MA teams in general can be more proactive when it comes to patient centricity
What is patient centricity?
One definition of patient centricity was provided by Dr. Olivier Menzel, Chairman and founder of the BLACKSWAN Foundation, at an event organized by Blue Matter in 2022. He said that patient centricity “means actively working in partnership with patients and members of the public to plan, manage, design, and carry out research. Research is carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’, or ‘for’ the public”. While this definition focuses on the research aspect, it is a useful guidepost because it clearly shows the importance of involving patients and caregivers in every step of the drug development journey as opposed to “just” delivering meaningful products or services.
In an environment governed by a truly patient-centric mindset, patients—as the end users of any therapy—are central stakeholders and decision-making partners in all key aspects of therapy development and use. For biopharma companies, delivering effectively on this ideal requires embedding the patient-centric mindset throughout the organization, rather than placing it in the “silo” of a dedicated team. Ultimately, every pharma company will need to clearly define what patient-centricity means to them, as well as how it should guide their organizational priorities and actions.
Why is it important?
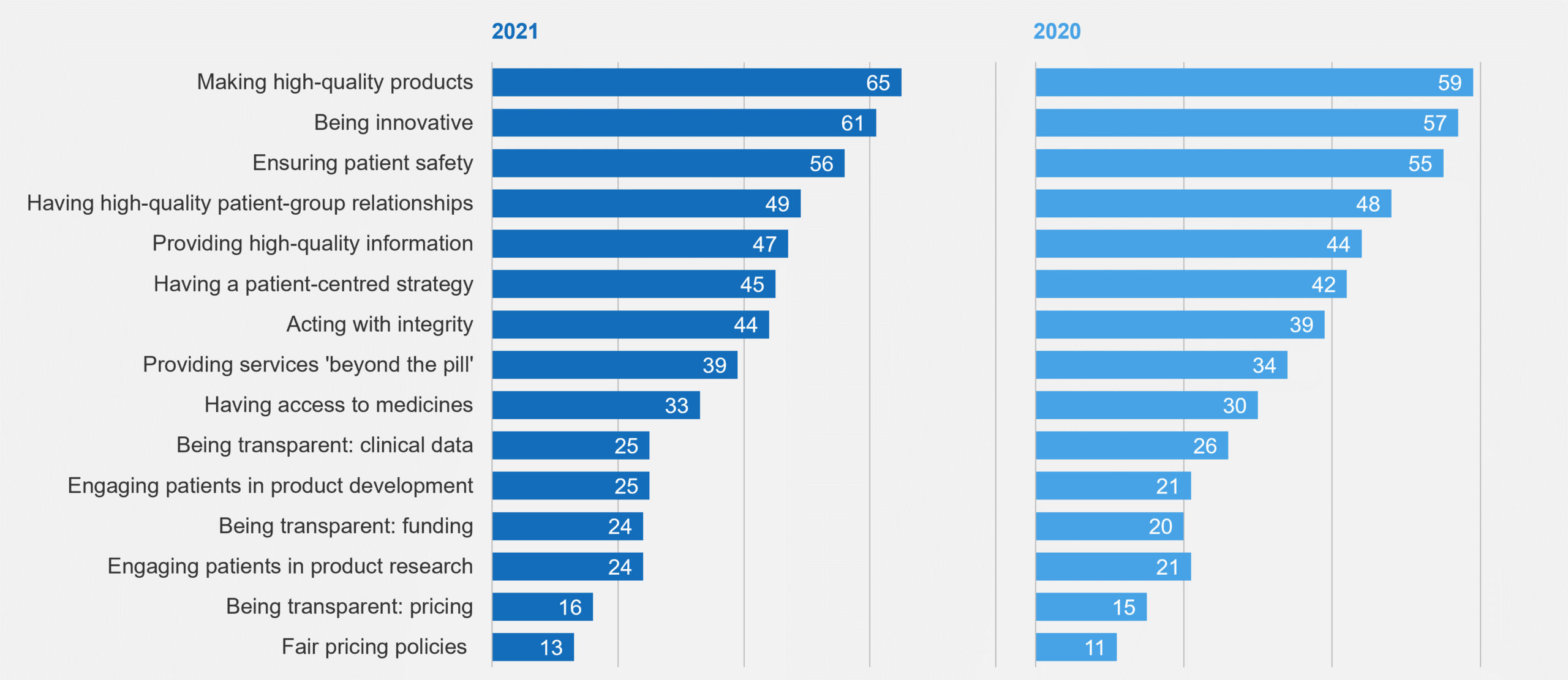
Aside from being the right thing to do because it can improve patient care and outcomes, ensuring patient centricity is also gaining in importance because patient expectations have changed. A 2021 survey conducted by PatientView asked patient groups and health campaigners to score biopharma companies on how well they have been delivering on patients’ needs and priorities. Figure 1 summarizes some of the results.
Figure 1: Percent of Respondent Patient Groups Stating “Excellent” or “Good” in 2021 PatientView Survey
While biopharma is perceived to deliver well on innovative and safe products and has made progress in providing high quality information, there is still significant room to address patients’ needs in multiple areas, such as including them in research and development and providing access to medicine.
Patients and patient groups aren’t the only ones with changing expectations. The US FDA’s patient-focused drug development initiative provides clear evidence that regulatory bodies (at least in the US) are driving this, as well. Via a series of guidance documents, the FDA provides input in four key areas:
- Collecting comprehensive and representative patient input
- Identifying what is important to patients
- Selecting or developing meaningful clinical outcomes assessments
- Incorporating outcomes assessments into endpoints for regulatory decision making
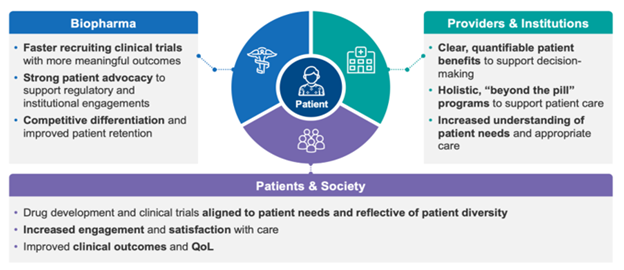
Every company that makes medicines should inherently be patient-centric. To truly address patient needs, drug developers need to understand their perspectives, give them a voice, and empower them in their journeys. Patient centricity brings benefits to stakeholders across the healthcare ecosystem, including biopharma companies. Figure 2 provides a few examples.
Figure 2: Benefits of Patient Centricity to Stakeholders Across the Healthcare Ecosystem
However, it’s no easy feat to become a patient-centric organization. Here a few things to consider:
- Patient centricity is a mindset that requires a cultural change to implement successfully (this takes time)
- Regulatory restrictions can limit certain types of patient engagements
- Heterogeneous country practices can make global patient centricity efforts difficult to implement
Now, let’s look at how some biopharma companies are addressing some of these challenges and putting the concept of patient centricity into action.
Examples of Patient-Centric Initiatives
Some readers may recall that in 2022, we produced a series of papers that described how the MA function has been evolving—and performing an increasingly key role—in a range of areas. These include:
- Insight Generation
- Medical Strategy
- Evidence Generation
- Medical Communications
- Stakeholder Engagement
The examples below are aligned to these areas. It’s true that some of the examples could easily be applied to more than one, but we’ve aligned each to a “best fit” area for simplicity’s sake.
Insight Generation
Understanding patient needs and caregiver needs in the moments that truly matter in the care continuum increasingly relies on active engagement from MA with patients and patient communities. This requires early and earnest cross-functional communication to ensure the patient perspective is incorporated throughout all aspects of therapeutics design, development, and delivery.
For example, we recently worked with a client who used patient surveys and a virtual panel to gather insights that could help them remove barriers to participation in clinical studies of a therapy for spinal cord injury. Through this engagement, the company was able to gather insights on how the patients’ symptoms impacted their daily lives, the challenges they faced when visiting the doctor, and what factors were most important to them when it came to participating in a study and using a therapy.
The surveys and panel provided a lot of useful information. In particular, patients focused on their need to maintain independence and the challenges they face with transportation to study / doctor visits. As a result of these insights, the team made important changes to study design and implementation that significantly improved their ability to address patient needs.
In a similar example, Novo Nordisk has developed a network of patient experts and advocates called Disease Experience Expert Panels (DEEPs). The members of these panels are primarily patients and caregivers but also include other patient experts such as patient advocacy representatives and colleagues at Novo Nordisk living with chronic disease. The integration of these different patient roles provides a rich and diverse array of perspectives. The panels are consulted when any new devices or therapies are developed to get deeper insights into what the customer really needs from the therapy.
Medical Strategy
For a biopharma company to be truly patient-centric, it must leverage a strategic approach that is cross-functional by necessity. In addition, the MA function must play a key role in developing, coordinating, and implementing such an approach.
Recently, a top-50 biopharma company with patient centricity as one of its corporate pillars took an honest assessment of how its teams defined patient centricity and how they put it into practice. The effort started with the MA organization. This assessment revealed key gaps between what patient centricity was in practice vs. what it should be in an ideal setting. It also helped to identify best practices that could be scaled across the entire organization.
From this, the company defined specific actions and programs that it needed to develop to close the gaps and achieve its objectives. It also developed a set of metrics to measure patient centricity and track progress against its goals as it implemented its overall strategy.
This type of approach—itself carried out with patient involvement and input—was instrumental in helping the company evolve from a reactive and relatively unguided approach to patient centricity to a proactive, strategically guided approach that was working towards defined goals.
Before building such a strategy, a company needs to think about how best to do it. For example, it may decide to anchor its analyses and insights on the patient journey, building its strategy around that central pillar, and measuring the success of its efforts based on the impact it has on patients at key points along the journey. It’s important to build the strategy around such a core concept, rather than trying to apply it later as an afterthought.
In the near term, approaches like the one described above will likely be repeated in company after company. It will become the standard way of doing business, and that’s a very positive thing.
Evidence Generation
Evidence can come in many forms and be generated by different functions within a biopharma company. For example, phase IIIb / IV studies, investigator sponsored trials (ISTs), real world evidence (RWE), and more can be used to support a range of needs that go far beyond clinical trials focused on regulatory approval. The MA function plays a critical role in developing such evidence. In doing so, it must consider a range of stakeholders, not least of which are patients.
AstraZeneca, for example, has worked with regulatory bodies to expand and incorporate the collection of patient symptom data in clinical trials. It expanded the patient details that were collected as part of its AURA3 trial comparing Tagrisso to platinum-based chemotherapy. The additional information tracked 28 different symptoms that patients reported during the trial, ranging from dry mouth to itchy skin. Collecting and providing this kind of information to patients helps them make more informed decisions about their treatment and helps them understand what to expect from their treatment experience.
Another interesting trend is the continuous growth of co-creation and collaboration between biopharma companies and patient communities to develop the evidence that is most meaningful to patients. Roche and Genentech have recently engaged with the Angelman Syndrome Foundation to design the FREESIAS biomarker study. Patients and caregivers are deeply involved in defining the areas in which they would like to see improvements, such as expressive communication, independence and self-care, sleep, fine motor skills, and gross motor skills. This study will be instrumental in developing clinical endpoints for future trials.
Medical Communications
Traditionally, external medical communications and education activities have been aimed at healthcare providers. However, as patients become more active partners in their own healthcare decisions, it’s important to provide education to them directly that meets them where they are.
This requires going beyond providing basic disease education and information on how to live well with the disease. Instead, it expands to include helping patients understand the clinical trial process, interpret scientific publications, and understand the data and evidence that will help them make more informed decisions about their care.
Physicians can play a role in distributing these materials to patients to help them be more confident and informed. Provider-focused and patient-focused educational resources should be beneficial to both audiences, helping to facilitate more informed communication between the two, and ultimately forging a better partnership around treatment plan decisions.
An example of this in action is the development of plain language summaries (PLS). The key concept consists of involving patients, patient advocacy groups, and caregivers to develop plain language summaries of clinical studies and other scientific research that is free of jargon, easy to understand, and accessible to the general public.
MA teams can develop and implement standards and processes for creating PLS for relevant scientific publications, and for making them accessible to patients. Jazz Pharmaceuticals, for example, created a website for delivering lay-level information—often via PLS—to those with small cell lung cancers.
Stakeholder Engagement
Stakeholder engagement includes partnering with patients and caregivers directly and engaging them indirectly to understand unmet needs, solicit insights, collaborate on goals, disseminate patient-friendly educational materials, and address other medical objectives. This can come in the form of patient / patient advocate conferences, patient advisory boards, patient representatives, and patient mentor programs.
In one example, Roche has created a role called the Patient Journey Partner (PJP). Per the role description, the PJP is an “empowered leader and the local face of Roche” for a given area. He or she “partners closely with all stakeholders directly touching the patient journey”—including providers, medical societies, institutions, and others—gaining a deep understanding of their needs. Key PJP goals are to connect Roche’s capabilities to those who need them and to “co-create meaningful solutions,” customized based on overall strategic goals and the capabilities of local healthcare system partners. The PJP role works across therapy and disease areas and is a patient journey strategy owner. Each PJP works with other PJPs to leverage ideas, capabilities, and synergies across healthcare ecosystems.
Sanofi Genzyme also operates a Journey Partners program, which was co-developed with multiple myeloma (MM) patients. It’s a bit different concept than the Roche program. These Journey Partners are MM patients who have lived with the disease. Their main mission is to educate other patients and their caregivers by sharing their stories and experiences. Through this program, Sanofi Genzyme has worked on better identifying patients’ needs and challenges, as well as the key questions they have. This understanding has led to the development of educational and service programs for healthcare providers and MM patients.
In another example, Ipsen worked with patients to co-design and launch a support website for patients with neuroendocrine tumors (NETs). The site is designed to support patients from pre-diagnosis through long-term living with NETs. Its development was informed by 900 respondents to patient questionnaires covering a range of needs and topics. Prior to launch, the site was validated with patients and healthcare providers. It provides a hub for information, tools, and support on a practical level.
Parting Thoughts
Clearly, patient centricity is more than a “buzzword” that gets repeated in articles and biopharma strategy documents. Instead, it’s rapidly becoming a guiding philosophy for biopharma companies and other members of the healthcare ecosystem. Those biopharma companies that effectively internalize the concept, infusing it throughout their organizations, will be the most effective in improving patient care via true patient centricity. For patients, the results will be more knowledge, higher confidence, greater convenience, better care, and improved outcomes.
Initially, those biopharma companies that master the concept will become even more integrated into the healthcare ecosystem and will build a significant competitive advantage. Over time, however, patient centricity will become the standard—and expected—way of doing business for biopharma.