In Part I of this series, we identified and described four key trends in oncology:
- Novel Drug Targets and Classes of Therapy
- Novel Drug Designs
- Tumor Biology-Based Regimens
- Diagnostic-Driven, Adaptive Regimens: Precision Medicine
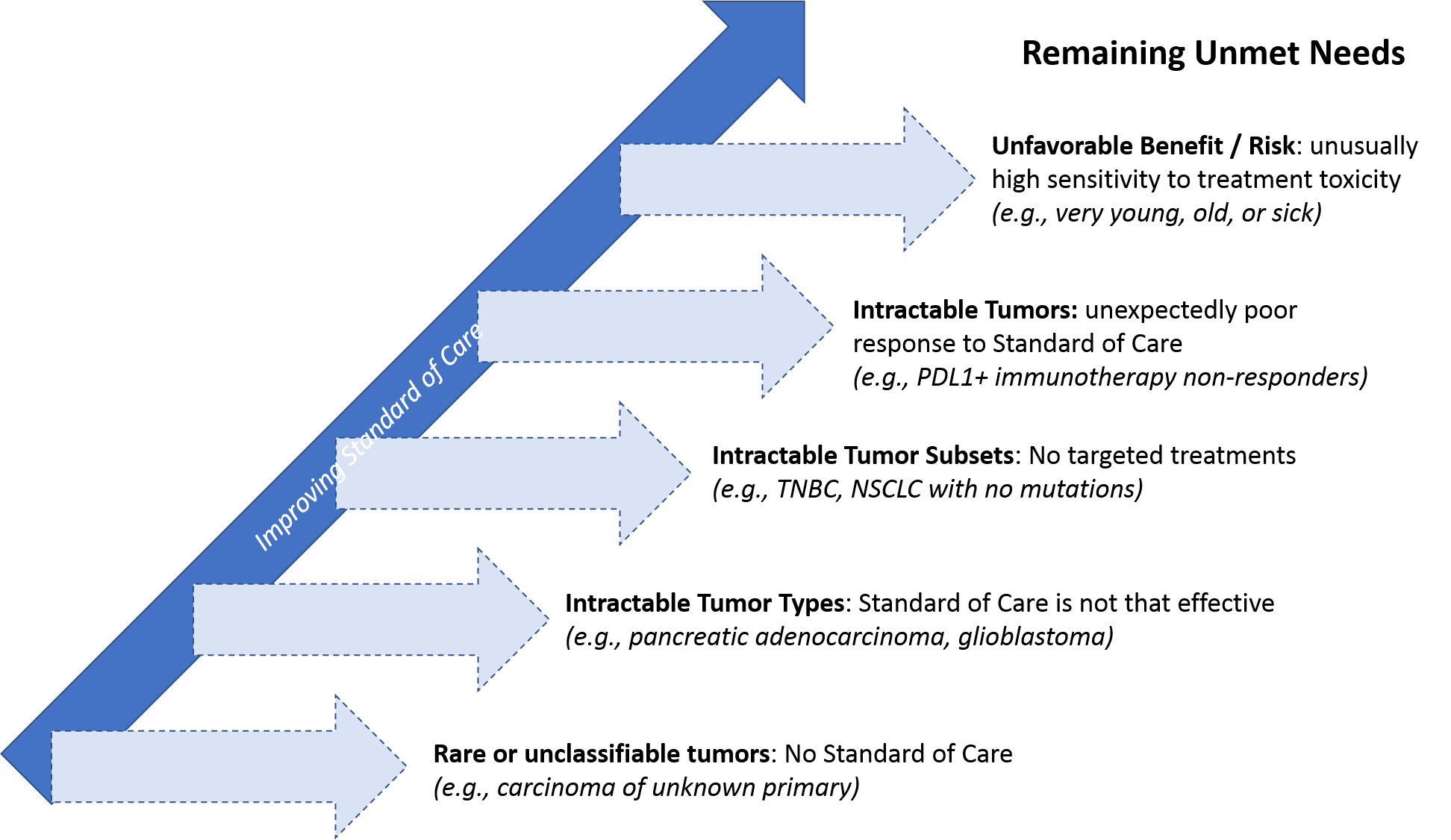
Despite the significant advances these trends represent, much unmet need remains in oncology. In this installment, we outline some of those key remaining unmet needs. We will also explore how oncology therapies are likely to advance in the next few years in order to meet these needs.
Unmet Needs
Even in highly segmented treatment settings like breast cancer or NSCLC—where numerous targeted drugs and personalized regimens are available—a subset of patients either has no targetable molecular alterations, their tumor does not respond to the personalized treatment, or there is a personalized treatment available but it’s too toxic for them (due to age, comorbidities, etc.).
The need is even higher in certain tumor types that have so far been intractable to targeted or personalized treatment. For example, in advanced pancreatic cancer and glioblastoma, despite decades of R&D investment, the standard of care has not advanced beyond maximum possible resection, radiation, and chemotherapy. As a result, most patients die of their disease within months. And across all tumor types, stage 4 (metastatic) cancer unfortunately remains a fatal rather than chronic but manageable condition for nearly all patients.
Finally, because cancer is such a heterogenous disease that can arise from any number of rare or even unique molecular alterations, some tumors are so rare or unclassifiable that no standard of care even exists for them. This can also be true of cancers identified at the metastatic stage where the primary origin is unknown (carcinomas of unknown primary, or CUPs).
Despite these challenges, we are confident that much progress will be made over the next 5 years and beyond. Specifically:
- An ever-improving understanding of tumor biology and therapeutic MOAs will drive novel and more efficacious drug designs and regimens
- The same scientific understanding that drives drug design AND the increased use of precision medicine AND a shift towards shared decision-making will inform more personalized and adaptive treatment strategies
What’s Next – Future Possibilities from Key Trends
Over the next 5 years we anticipate continued success in evolving drugs and regimens to meet these needs. From a target perspective, there are a few key pathways with novel targets approaching approval.
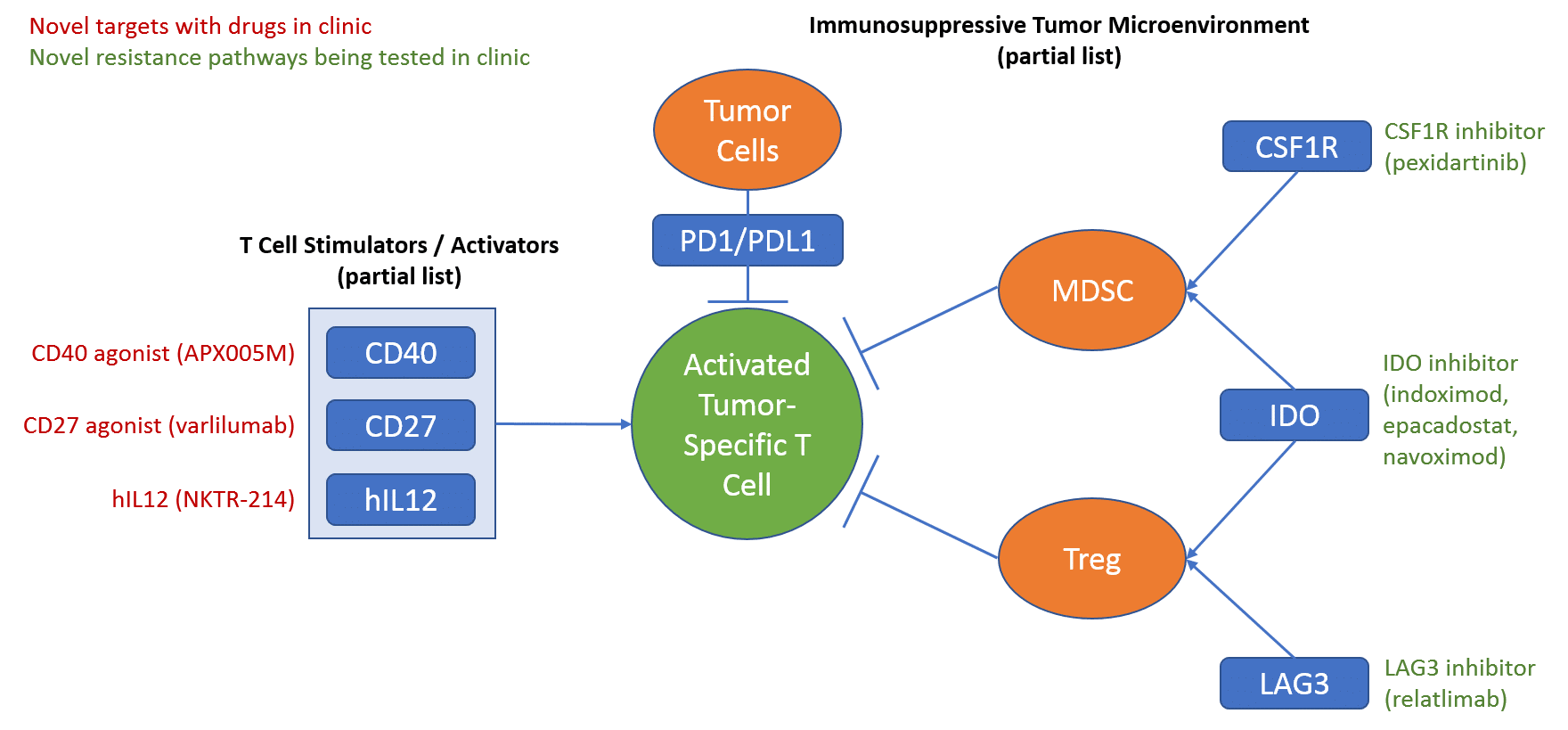
The first major class of targeted therapies are those directed at targets involved in immunotherapy. In practice, these will likely be used to either boost efficacy or address resistance to the already marketed checkpoint inhibitor drugs (PD1 and PDL1 targeted drugs).
After the approval of the PD1/PDL1 checkpoint inhibitors, there has been extensive investment in numerous other targeted approaches with potential to further improve response to the checkpoint inhibitors. Over the past few years, many of these first-generation drugs have failed to demonstrate clinical efficacy. However, several approaches are still in clinic and showing some encouraging signs of efficacy.
One promising approach for boosting checkpoint inhibitor efficacy is to target immune cell activators using agonist drugs that bolster their activity. The other angle (targeting resistance mechanisms) is via the more traditional inhibitor approach of blocking the activity of proteins that enable tumors to evade the immune system. Over the next few years we anticipate further clarity on which specific targets and drugs are truly efficacious, and importantly, which regimens and settings they should be used in for maximal benefit.
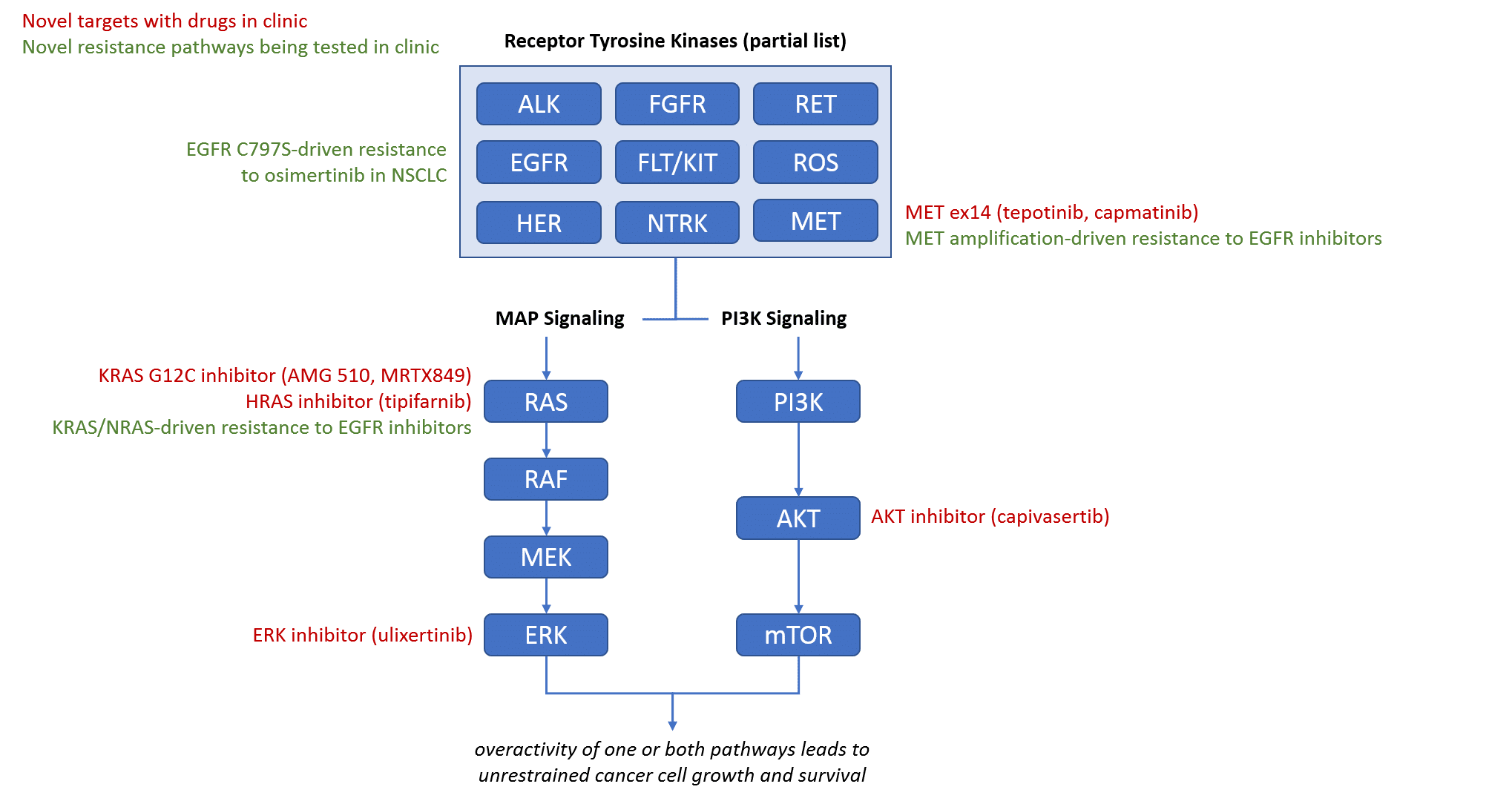
The other major class of targeted therapies are those directed at targets involved in the Receptor Tyrosine Kinase-driven signaling pathways that are frequently inappropriately active in tumors, resulting in uncontrolled tumor cell growth. Specifically, the MAP signaling and PI3K signaling pathways both have key targets with promising drugs currently in clinic. In addition to novel targets, there are also multiple strategies already being tested in clinic to address known resistance mechanisms to existing drugs, sometimes by mixing and matching already approved drugs.
With the launches of additional new drugs and classes of therapy, we anticipate that tumor biology-based regimens will continue to advance. In addition to the likely expansion of the PARP inhibitors as both a primary treatment and a maintenance therapy into additional germline BRCA tumor indications, we expect that the appropriate sequencing of the checkpoint inhibitor therapies will be clarified, introducing these drugs to the standard of care beyond just first line or second line therapies (e.g., neoadjuvant or adjuvant maintenance treatments).
Depending on which of the novel immunotherapy targets proved successful in clinic, we also anticipate integration into combination regimens with the checkpoint inhibitors. These may or may not be biomarker-dependent as part of the rationale for these combinations is that they may be able to induce response to a checkpoint inhibitor even in a likely non-responder.
Similarly, the introduction of additional receptor tyrosine kinase pathway inhibitors will likely lead to new targeted combinations, hopefully in some of the more challenging tumor types like pancreatic (where KRAS is almost always mutated). We also expect additional resistance targets to extend the treatment options for EGFR, HER, and ALK driven tumors in later lines of therapy.
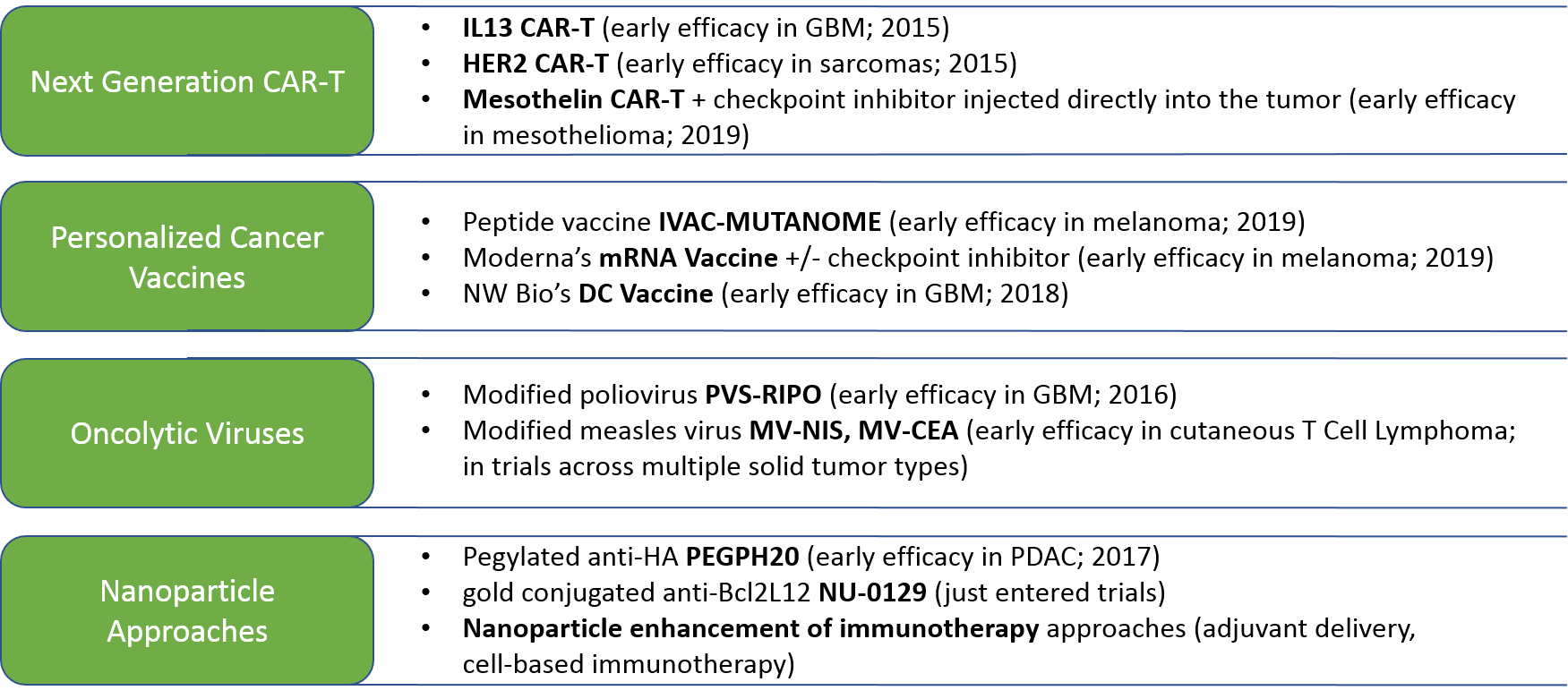
In parallel, we anticipate continued evolution and success of drug and regimen design strategies, in particular the cell-based immunotherapies as discussed in our previous series. In addition to the cell-based immunotherapies, we expect the improvement of existing—and emergence of new—therapeutic designs, including:
Finally, we anticipate a continued shift towards truly personalized and more adaptive regimens, as we learn more about the most effective use of both existing and novel drugs and regimens. The impact of this trend depends on successful development and clinical uptake of broad genomic tests, particularly the blood-based or other liquid biopsy ones that enable frequent testing to monitor therapeutic response and the emergence of resistance mutations.
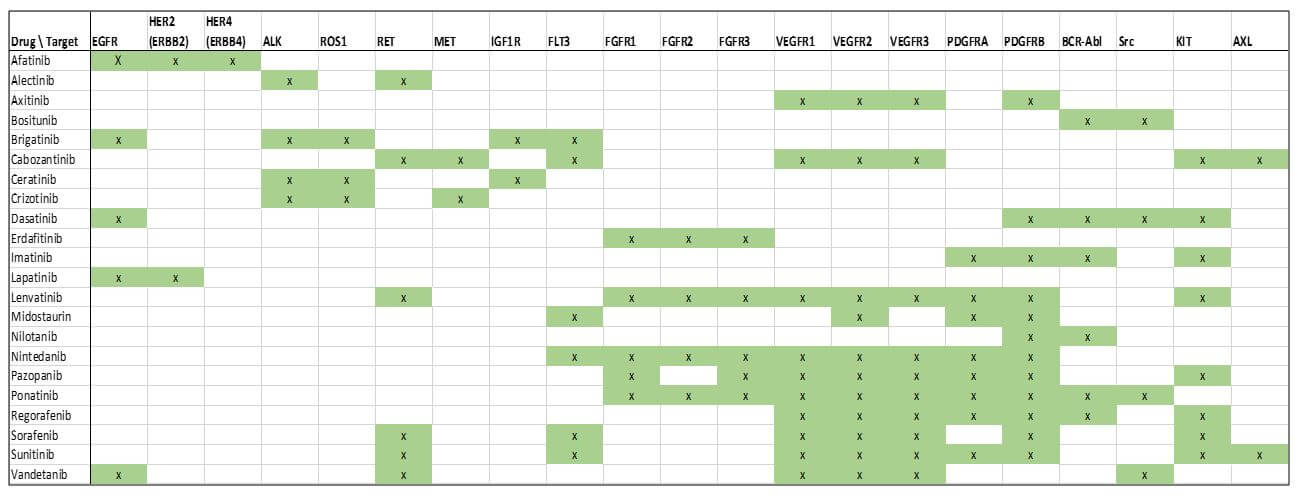
In parallel, we expect a continued deeper understanding of which molecular alterations and patterns of molecular alterations are the best predictors of response, as well as lack of response and toxicity. One particular arena where we may see rapid advances is with the protein kinase inhibitors. Because some targets of these molecules are closely related to one another, there is frequently a halo of efficacy for these drugs towards not just their primary target, but multiple other targets as well.
Interestingly, different approved drugs have different patterns of targeting, showing additional potential for more targeted application based on variations in molecular alterations for each individual tumor. Since these drugs are already approved, there may be rapid advancement in their use both individually and as part of regimens as tumor molecular testing becomes more available and practical.
![]() Taken together, there is much to be hopeful in the near-term future for oncology, as we continue to push the standard of care towards diagnostic-driven, adaptive regimens in broader patient populations. In our next installment, we will discuss the requirements for pharmaceutical and diagnostic companies to lead and succeed in this future landscape.
Taken together, there is much to be hopeful in the near-term future for oncology, as we continue to push the standard of care towards diagnostic-driven, adaptive regimens in broader patient populations. In our next installment, we will discuss the requirements for pharmaceutical and diagnostic companies to lead and succeed in this future landscape.