Cell therapies have come a long way and they continue to advance in the fight against many cancers. From their start in acute lymphocytic leukemia (ALL), they expanded into non-Hodgkin’s lymphoma (LBCL, FL, and MCL), and moved up from a third-line treatment to second line. They’ve been approved in fifth-line + multiple myeloma (MM) and are likely to advance there, as well, perhaps even to first-line therapy.
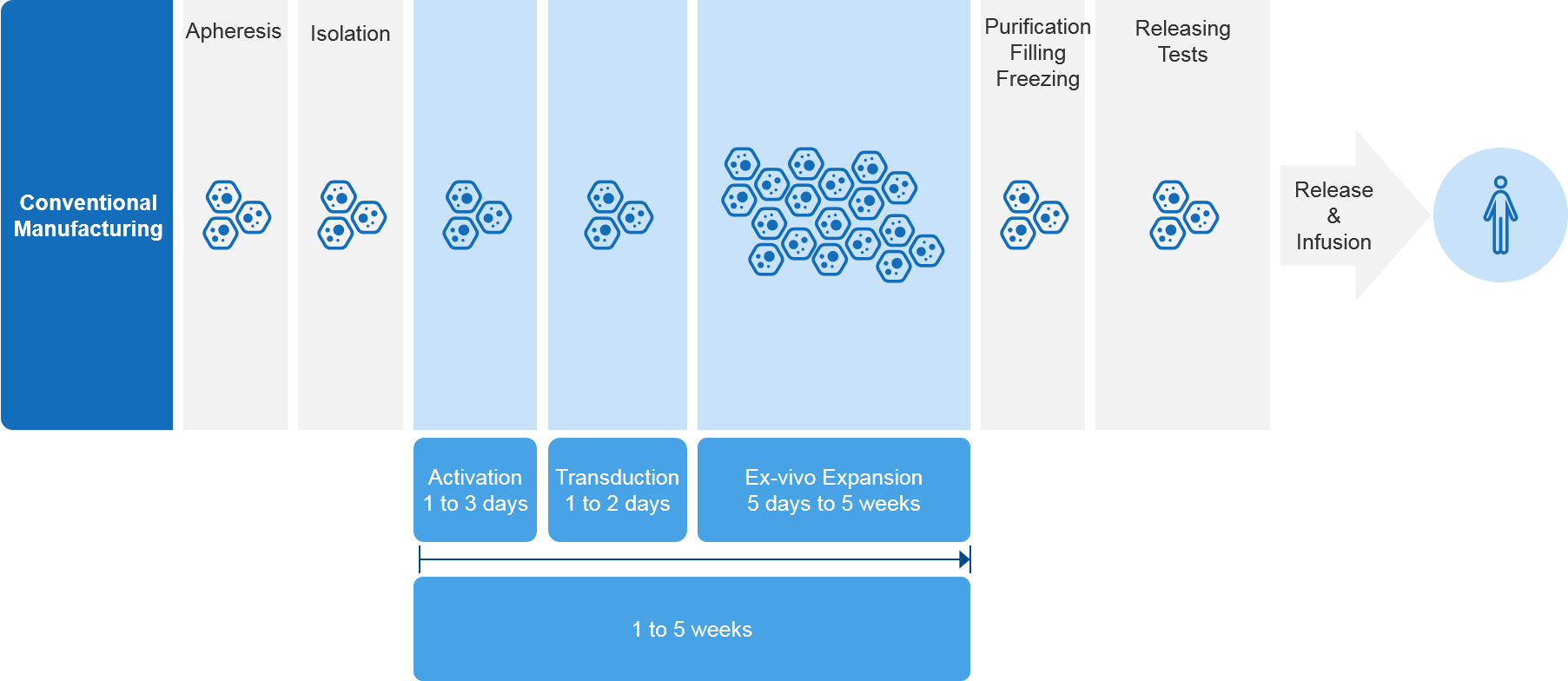
The expansion of cell therapies offers promising new alternatives for patients, but this advance is not without its challenges. A key challenge relates to their highly complex and labor-intensive manufacturing process and supply chain bottle necks, which can force patients to wait extended periods for therapy (see Figure 1).
Figure 1 – Current CAR-T Manufacturing Timeline (excluding transport times)
As demand for cell therapies increases, this places further strain on their manufacturing sites and supply chains, exacerbating the situation. For example, in ALL and DLBCL (which are relatively small indications compared to MM), patients must wait two to three weeks for therapy. In MM, patients often face considerable wait times of up to two months. BCMA CAR-T therapies are only available in a small number of centers, with limited manufacturing slots (1-2) coming open each month.[1]
In short, demand is growing faster than the manufacturing and supply chain’s ability to keep up and scaling up additional capacity is not a simple undertaking. In the near- to mid-term, the patients who need these therapies the most are more likely to face long waits. Additionally, the companies who develop these therapies will face an uphill battle when it comes to maximizing their commercial potential.
So, where do we go from here? This topic was discussed at the recent meeting of the American Society of Hematology (ASH) in December of 2022. In this article, we address several of the potential solutions that are most commonly cited, offering a brief explanation of each and what we think the future might hold.
The Potential Solutions at a Glance
Cell therapy manufacturers continually evaluate manufacturing and supply chain processes to identify ways to improve them and enhance efficiency. Basically, four key approaches are being explored:
- “Rapid manufacturing” – “Rapid manufacturing” techniques aim to reduce overall processing time from several weeks down to just one or two days.
- Decoupling cell therapy production from viral vectors – The use of viral vectors, while effective, has been a bottleneck in supply chains,[2] causing delays in cell-therapy production. Developing gene delivery methods that do not rely on viral vectors can potentially streamline supply chains and reduce manufacturing timelines.
- Allogeneic cell therapies – Allogeneic therapies eliminate the need for a bespoke manufacturing process for each patient and may help transform cell therapy as closely as possible to an off-the-shelf, readily available product.
- Increased automation – Cell therapy is highly labor intensive. Reducing the amount of human labor in cell therapy production can potentially increase efficiency and streamline the process.
Below, we take a deeper look at each of these potential solutions. We share any relevant and recent information on each while offering some additional insights when appropriate.
Rapid Manufacturing
At the ASH annual meeting, both Novartis and Gracell showcased clinical datasets for rapidly manufactured CAR-T therapies. Each company leverages a platform designed to shorten the manufacturing time from weeks to days.
Novartis T-Charge™ Platform
With the T-Charge platform, CAR-T cell expansion occurs primarily within a patient’s body (in-vivo), eliminating the need for an extended culture time outside of the body (ex-vivo). T-Charge preserves T cell stemness—the ability to self-renew and mature—which results in a product containing greater proliferative potential and fewer exhausted T-cells. The platform’s unique characteristics may lead to better and more durable responses, improved long-term outcomes, and reduced risk of severe adverse events.
Novartis highlighted YTB323, a CD19 targeting CAR-T that is the product of their T-Charge™ manufacturing platform. Novartis demonstrated that in a process that takes only two days for manufacturing, they are able to preserve the T-cell stemness and generate 83% overall response rate (ORR) and 73% complete response (CR) at a dose level that is 25 folds lower than Kymriah®.
Table 1: YTB323 (T-Charge) vs. Kymriah®
| YTB323 (Rapcabtagene Autoleucel) (T-Charge) | Kymriah | |
|---|---|---|
| Manufacturing Process | CAR-T cell expansion occurs primarily in vivo | CAR T cell expansion occurs ex vivo following a general CAR-T manufacturing process |
| Dose | 12.5 x 106 CAR+ cells | A single infusion of 5 x 108 viable CTL019 transduced cells, which will be administered via intravenous infusion |
| Number of Patients | 28 | 115 |
| Results: | ||
| CR | 73% | 39% |
| ORR | 83% | 53% |
Gracell FasTCAR Platform
FasTCAR is very similar to T-Charge. It combines a three-step process (activation, transduction, and expansion) into a single step called “concurrent activation transduction.” Due to minimal ex vivo time, FasTCAR T-cells remain young and show enhanced proliferation and tumor clearance activities in preclinical studies. This enables lower cell dosage and eliminates the need for ex vivo expansion, which instead happens in vivo.
Table 2: BCMA/CD19 Dual-Targeting FasTCAR-T Cells vs. ABECMA® in Relapsed/Refractory (R/R) Multiple Myeloma
| BCMA/CD19 Dual-Targeting FasTCAR-T Cells (GC012F) (In consolidation NDMM) | ABECMA in R/R MM (At least 4 lines of treatment) | |
|---|---|---|
| Manufacturing Process | 22 to 36 hours (Single Step manufacturing Process) | 28 day process: Ex-vivo engineering and expansion |
| Dose | 1x105/kg, 2x105/kg, or 3x105/kg (DL2: 140x105 @ 70 kg) (NDMM) | 300 to 460 × 106 CAR-positive T cells (R/R MM) |
| Results: | ||
| CRR | 69% | 29% |
| ORR | 100% | 72% |
These datasets appear to demonstrate that efficacy is not being compromised in this process, essentially delivering a highly efficacious product over a shorter duration.
Rapid manufacturing techniques can shorten the wait for patients and may end up being a significant part of the overall solution to the current capacity challenge. However, these datasets only account for the manufacturing and do not provide a more comprehensive view of the total time from the collection of a patient’s cells until they are infused back to the patient as CAR-Ts.[3]
Decoupling Cell Therapy Production from Viral Vectors
Viruses are extremely efficient at introducing genetic material into host cells. During the cell therapy manufacturing process, viral vectors are often used to:
- Introduce new genetic material into the cell
- Promote integration of the material into the cell genome
Thereby generating a stable expression in all the cells that will expend in that culture.
However, viral vector production does not seem to meet the rapidly expanding demand for cell therapy manufacturing. As a result, several approaches are being developed that aim to decouple cell therapy manufacturing from the need for viral vectors. Those approaches, if successful, may streamline manufacturing and resolve a key supply chain bottleneck.
One overall approach involves the use of transposon-based systems such as PiggyBac, Sleeping Beauty or Tol2, which are usually introduced into the cell via electroporation and rely on the natural ability of these DNA fragments to translocate across different areas in the genome to introduce new genetic material into cells.
These approaches were used to generate several CAR-T products harboring the CD19-targeting CAR. Overall, efficiency was somewhat limited, with a relatively low yield of transduction and even a lower yield of incorporation into the genome and the generation of a CAR-T.
In addition, safety concerns were raised with this approach. In a recent phase I trial, lymphoma arising from piggyBac-transfected CAR T cells was reported in two of nine patients with relapsed or refractory B-cell malignancies who had achieved complete remission after product infusion. The analysis of the lymphoma samples showed an increase in the transgene copy numbers but with no integration in typical oncogenes.[4] These findings reiterate the need for better controlled and precise gene transfer modalities.
Other approaches are exploring the use of mRNA or CRISPR delivered by liposomal nano particles to introduce genetic manipulations into cells. A CD-19 targeting CAR-T cell generated using a CRISPR system that was delivered into the cells via electroporation generated an ORR of ~81% in R/R B-cell lymphoma patients.[5]
The CARAMBA trial in multiple myeloma patients has been recently launched. It is using mRNA encoding the hyperactive SB100X transposases in conjunction with an anti-slam family member 7 (SLAMF7)-encoding CAR transposon.
Table 3: Comparing Viral Vectors and Key Transposon Vectors
| Transduction Method | Viral Vectors | Transposon Vector | Transposon Vector |
|---|---|---|---|
| Specifications | LV/RV Vectors | Sleeping Beauty | PiggyBac |
| Efficiency | Very High | Low-Medium | Medium |
| Manufacturing Cost | High | Moderate | Moderate |
| Vector Genetic Material Capacity | +/-10kb | 5kb to tens of kb | Hundreds of kb |
Allogeneic Cell Therapies
As mentioned earlier, allogeneic cell therapies use donor cells, eliminating the need to first extract cells from the patient. Therapy is essentially delivered “from stock” and while there is a matching process, allogeneic therapies involve fewer production steps, are simpler to manufacture, and are much closer to an “off-the-shelf” product.
However, existing allogeneic CAR-Ts in clinical development are currently falling short of the efficacy benchmarks set by recently approved—but more complex—autologous CAR-T therapies. Table 4 compares the clinical efficacy of clinical-stage allogeneic CAR-Ts versus recently approved autologous CAR-T therapies.
Additionally, allogeneic CAR-T therapies face unique challenges due to the risk of graft versus host disease (GVHD) and allorejection, either of which can cause serious adverse events and result in decreased drug efficacy. Most development techniques of allogeneic CAR-T products currently under investigation are guided by the hypothesis that allogeneic CAR-Ts require gene editing and alterations to “hide” them from the patients’ immune systems to limit or prevent the possibility of GVHD or allorejection.
Table 4: Efficacy of Clinical-Stage Allogeneic CAR-T Therapies vs. Approved Autologous CAR-T Therapies
| Generic Name | ADI-001 | lisocabtagene maraleucel | ALLO-715 | ciltacabtagene autoleucel |
|---|---|---|---|---|
| Brand Name | - | Breyanzi | - | CARVYKTI |
| Manufacturer | Adicet | Bristol Myers Squibb | Allogene | Janssen |
| Product | Allo γδ CAR-T | Auto CAR-T | Allo CAR-T | Auto CAR-T |
| Target | CD20 | CD19 | BCMA | BCMA |
| Indication | B-cell malignancies | LBCL | RR MM | RR MM |
| Phase | 1 | FDA Approved | 1 | FDA Approved |
| Clinical Trial | GLEAN-1 | TRANSFORM | UNIVERSAL | CARTITUDE-1 |
| Patient Population Represented in Results | 9 | 184 | 21 | 97 |
| CR (%) | 78% | 66% | 27%, 17%** | 78% |
| mEFS (months) | - | 10.1 | - | - |
| mPFS (months) | - | 14.8 | - | NR* |
| ORR (%) | 78% | 80% | 64%, 67%** | 98% |
| ORR at 6 Mo FU (%) | 18% | - | - | - |
* NR: Not reached; **Based on lymphodepletion dose levels FCA39 and FCA60, respectively.
Increased Automation
Currently, cell therapy manufacturing is a highly hands-on, labor-intense process. It requires a substantial and well-trained labor force. Introducing a higher degree of automation can reduce the dependency on skilled labor, streamline the process, and potentially shorten manufacturing times. In addition, process automation can improve product consistency and quality by reducing the potential for human error.
A fully automated process can also utilize the manufacturing footprint more efficiently, allowing a manufacturer to process more patient samples simultaneously. Various systems are being developed to provide “hands-free” cell manufacturing “in a box.”[6] Some of these include
- Lonza’s Cocoon modular cell therapy manufacturing platform
- Miltenyi Biotec’s CliniMACS Prodigy platform
- Cellares’ Cell Shuttle
However, for these automated devices will need to integrate in-line QC in order to truly impact the production timelines.
Parting Thoughts
In the cell-therapy space, the product is closely linked to the process. Therefore, there is limited ability to dramatically change the manufacturing process once a pivotal study is initiated. Therefore, cell-therapy companies need to commit to their manufacturing processes ahead of time. Because the technologies that aim to streamline cell-therapy production are still at an early development phase, cell therapy companies need to assess how to incorporate them while mitigating development risks for their products.
Table 5: High-Level “Pros and Cons” of Potential Solutions
| Solution Strategy | Pros | Cons |
|---|---|---|
| Rapid Manufacturing |
|
|
| Decoupling Cell Therapy from Viral Vectors |
|
|
| Allogeneic Cell Therapies |
|
|
| Increased Automation |
|
|
Some considerations to assess these approaches may include:
- Scientific – the level of validation that these approaches have demonstrated in the past and potential efficacy / safety tradeoffs
- Market – the unmet need(s) in the market these technologies are addressing
- Strategic – the partnership model and risk mitigation between the cell therapy company and technology holders for the various approaches
Table 4 References:
Adicet:
- https://ash.confex.com/ash/2022/webprogram/Paper157400.html
- https://www.evaluate.com/vantage/articles/events/conferences-trial-results/ash-2022-adicet-has-case-deja-vu
Allogene UNIVERSAL trial:
- https://ir.allogene.com/static-files/ed733aeb-fa9f-4c70-9220-f7e726fdc6c4
- https://ash.confex.com/ash/2022/webprogram/Paper158231.html
Breyanzi:
- https://www.fda.gov/media/159602/download
- https://news.bms.com/news/details/2022/US-FDA-Approves-Bristol-Myers-Squibbs-CAR-T-Cell-Therapy-Breyanzifor-Relapsed-or-Refractory-Large-B-cell-Lymphoma-After-One-Prior-Therapy/default.aspx
CARVYKTI:
- https://www.cancer.gov/news-events/cancer-currents-blog/2022/fda-carvykti-multiple-myeloma
- https://www.jnj.com/longer-term-data-from-cartitude-1-study-demonstrate-continued-deep-and-durable-responses-to-carvykti-ciltacabtagene-autoleucel-in-heavily-pretreated-patients-with-relapsed-or-refractory-multiple-myeloma
Adicet:
- https://ash.confex.com/ash/2022/webprogram/Paper157400.html
- https://www.evaluate.com/vantage/articles/events/conferences-trial-results/ash-2022-adicet-has-case-deja-vu
Allogene UNIVERSAL trial
- https://ir.allogene.com/static-files/ed733aeb-fa9f-4c70-9220-f7e726fdc6c4
- https://ash.confex.com/ash/2022/webprogram/Paper158231.html
Breyanzi:
- https://www.fda.gov/media/159602/download
- https://news.bms.com/news/details/2022/US-FDA-Approves-Bristol-Myers-Squibbs-CAR-T-Cell-Therapy-Breyanzifor-Relapsed-or-Refractory-Large-B-cell-Lymphoma-After-One-Prior-Therapy/default.aspx
CARVYKTI:
End Notes:
[1] Liu, Angus, Cancer center leaders lay bare CAR-T makers’ struggles—and an unexpected laggard, Fierce Pharma, Dec. 20, 2022, https://www.fiercepharma.com/pharma/johnson-johnson-bristol-myers-kite-pharma-car-t-cell-therapy-struggle-sloan-kettering
[2] Liu, Angus, Bristol Myers hits CAR-T manufacturing bottleneck as Abecma demand outstrips supply, Fierce Pharma, July 28, 2021, https://www.fiercepharma.com/manufacturing/bristol-myers-hits-car-t-manufacturing-bottleneck-as-abecma-demand-outstrips-supply
[3] Plieth, Jacob, Ash 2022 – fast production fails to cure Car-T’s problem, Evaluate Vantage, Dec. 11, 2022, https://www.evaluate.com/vantage/articles/events/conferences-trial-results/ash-2022-fast-production-fails-cure-car-ts
[4] Abou-El-Enein M, Elsallab M, Feldman SA, Fesnak AD, Heslop HE, Marks P, Till BG, Bauer G, Savoldo B. Scalable Manufacturing of CAR T cells for Cancer Immunotherapy. Blood Cancer Discov. 2021 Sep;2(5):408-422. doi: 10.1158/2643-3230.BCD-21-0084. Epub 2021 Aug 3. PMID: 34568831; PMCID: PMC8462122. https://pubmed.ncbi.nlm.nih.gov/34568831/
[5] Hu, et al, High Safety and Efficacy of CRISPR-Based Non-Viral PD-1 Locus Specific Integrated Anti-CD19 CAR-T Cells (BRL-201) in Treating Relapsed or Refractory Non-Hodgkin’s Lymphoma: First-in-Human Phase I Study, Blood (2022) 140 (Supplement 1): 7491–7492. https://ashpublications.org/blood/article/140/Supplement%201/7491/488600/High-Safety-and-Efficacy-of-CRISPR-Based-Non-Viral
[6] Tay, Andy and Reno Lim, Rachel, Cell Therapy Manufacturing Shifts to Automatic, Genetic Engineering & Biotechnology News, Oct. 4, 2021, https://www.genengnews.com/topics/bioprocessing/cell-therapy-manufacturing-shifts-to-automatic/