As we described in part I of this series, gene therapies are complex, as are the commercial planning processes associated with them. In part II, we narrowed our focus, diving into the myriad complexities surrounding gene therapy supply chains, including manufacturing, packaging, labeling, logistics, and so on.
In this paper, we focus on another critically important aspect of gene therapies: the cost drivers. As everyone in this field knows, gene therapies are expensive. What might be less obvious are the specific elements that drive the costs for gene therapies. Here, we identify those elements and highlight potential factors that can, over time, ease the pressure.
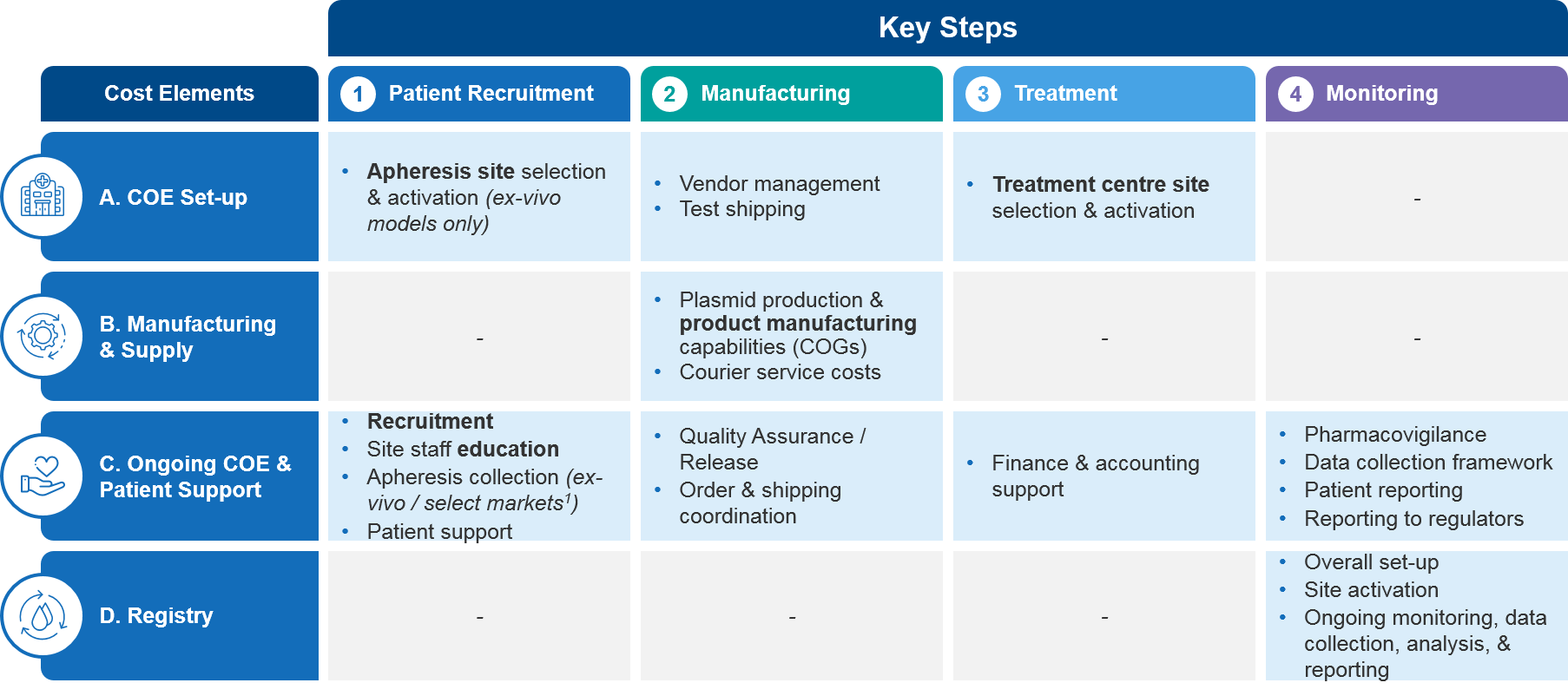
Gene therapy commercialization comes with some costs that are different from more conventional pharmaceuticals. To facilitate our overview, it’s helpful to link them to the four key steps in the process for administering ex vivo therapies, which we described in part I (see Figure 1):
- Patient Recruitment
- Manufacturing
- Treatment
- Monitoring
Figure 1: Key Steps in Ex Vivo Therapy Administration and the Associated Cost Elements
Below, we provide a qualitative description of the cost drivers for each step, as well as some insights into how these costs might be addressed over time.
Center of Excellence (COE) Set-Up
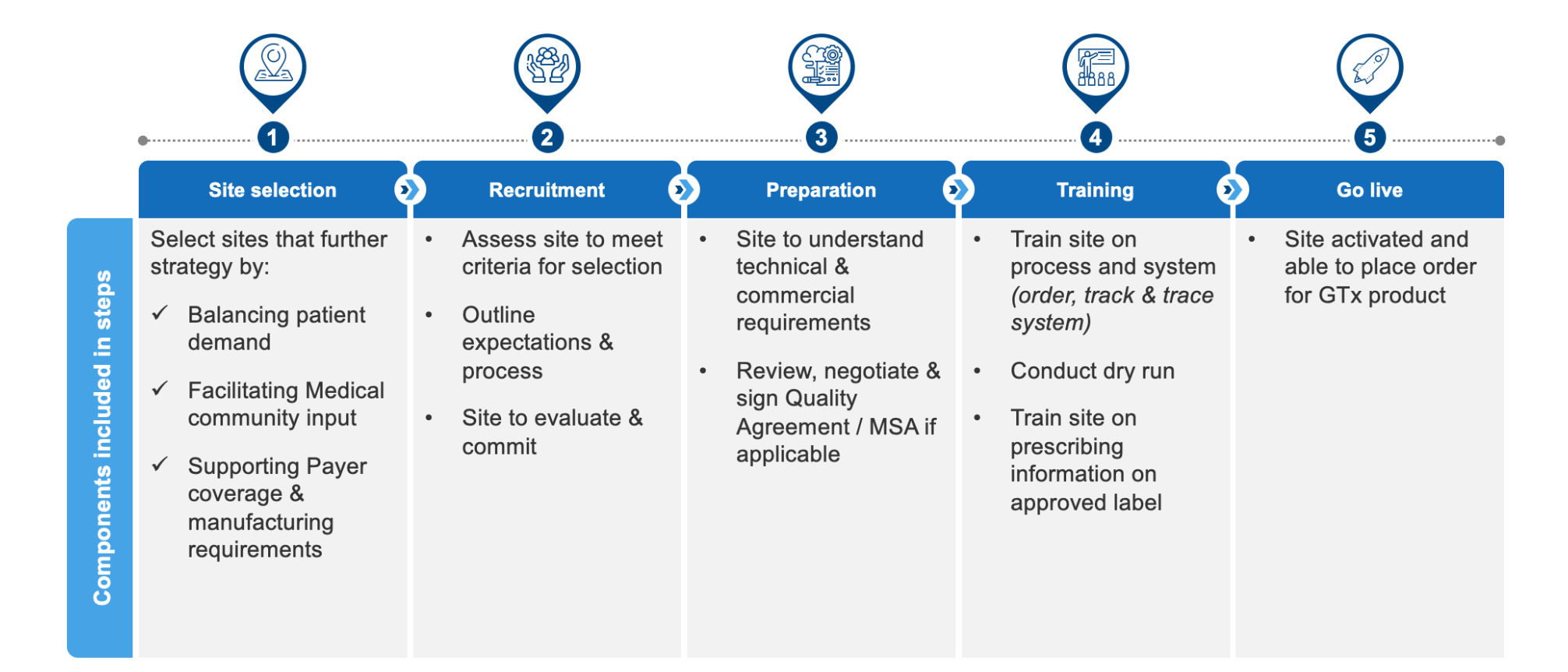
Setting up a COE is a lengthy, multi-step process that starts with site selection and culminates when the site goes live. Figure 2 provides an overview.
Setting up a single COE for an ex vivo therapy (i.e., one that includes apheresis and infusion capabilities) can take up to 800 person-hours. The process is considerably simpler for an in vivo therapy, in which the COE will handle infusions only. In that case, the FTE requirements are cut by more than two-thirds. Other factors that contribute to the number of hours required are whether the site has previous experience with a gene therapy, owns the blood bank services, and can assign sufficient resources (among others).
Figure 2: Key Steps in Setting Up a COE for an Ex Vivo Gene Therapy
More than half of the costs associated with COE set-up, particularly when dealing with ex vivo therapies, are related to the commercial and manufacturing functions. For example, commercial personnel have to map and rank potential sites, assess their likely pros and cons, contact the sites and conduct initial meetings, conduct training, and coordinate the process to ensure that all goes live in an orderly manner. Manufacturing and Quality have to assess the supply chain feasibility, help set up the process at the site, lead much of the technical training, and conduct audits. For any given therapy, these costs are at their greatest for an initial launch, and may not be as applicable to subsequent generations of the initial therapy.
With an increasing number of Advanced Therapy Medicinal Products (ATMPs) coming to the market, it’s likely that the processes associated with COE set-up will begin to standardize. This should have a positive impact on set-up costs over time. In addition, the potential use of JACIE accreditation as a standard may reduce or eliminate the need for audits by each manufacturer. Finally, increased standardization of things like storage containers, labels, consents / authorizations, and training should help drive efficiency and improve cost-effectiveness.
Manufacturing and Supply
The manufacturing and supply elements are, unsurprisingly, the largest cost drivers associated with gene therapies. Due to their bespoke nature, ex vivo therapies carry much higher manufacturing and distribution costs than in vivo therapies, and can rise beyond $500,000 per patient in some cases.
Over time, however, manufacturing costs should come down as processes are streamlined and novel technologies become more widespread. As these therapies proliferate, economies of scale should enable manufacturers to make more efficient use of resources and labs.
Biopharma companies should consider a few key factors regarding these cost drivers:
- It’s important to begin optimizing manufacturing processes early – The infrastructure developed during the clinical development process will become the basis for the commercial infrastructure. When developing processes to support clinical development, a company should think beyond the development stages and work to optimize manufacturing for a full-scale environment.
- Flexible contracting can be effective at controlling costs – Gene therapies are relatively new, so contract manufacturing organizations (CMOs) are also learning how to do this effectively. As a result, the full suite of services may not be available in a lot of cases. However, if a biopharma company is able to secure a suitable CMO, then it should explore innovative and flexible contracting arrangements that would enable rapid up- or down-scaling of manufacturing on an as-needed basis.
- In-sourcing may also be an excellent strategic pathway – For some biopharma companies, it may make more sense to in-source gene therapy manufacturing. It ultimately comes down to a question of scale and the geographic distribution of its patients. If a company’s therapy (or upcoming portfolio of therapies) will bring sufficient scale, then it may be more cost-effective to invest in creating regional in-house manufacturing hubs.
Ongoing COE and Patient Support
On an ongoing basis, patients who benefit from gene therapy also receive significant healthcare services that relate to the therapy. Specific to the therapy’s delivery and follow-up, however, there are a few ongoing costs that a biopharma company must consider.
These costs mainly center around the following items:
- Local Affiliate Support – Educate referring physicians, support referrals, coordinate COEs, and resolve potential issues
- Customer Service – Place orders and coordinate with the COEs, shippers, and others
- Supply – Monitor and coordinate shipments
- Manufacturing – Conduct all processes related to the reception, transduction, quality assurance, and release of product
- Finance – Process invoices, organize debt collection, and accounting
- Shipping – Manage the bi-annual or annual shipping and analysis of patient samples
- Medical Affairs and RWE – Provide information and education to COE staff and support registries (see below)
- Pharmacovigilance
As the gene therapy space matures, it is likely that process automation will help reduce these costs. Likewise, tighter networking with and among COEs can also have a positive impact.
Registry Set-Up
With both in vivo and ex vivo therapy models, a registry is typically required by regulators to track patients and their results over time. This provides insights regarding a therapy’s durability, potential long-term side-effects, and more. Some registries will track patients for as long as 15 years.
There are significant costs associated with setting up and maintaining these registries. For any given site, the initial set-up and first-year maintenance costs can run to $150,000 or more. These costs can include registry protocol development and approval, site training and start-up, ICF reviews, monitoring, database oversight, and project management.
The costs continue beyond the first year. During the full course of years 2-15, costs can total several hundred thousand dollars per site. This covers many of the same items listed above—as they recur on a regular basis—as well as ongoing communication costs and local contract research organization (CRO) oversight (assuming a CRO is used).
In one interesting example, Kite Pharma has collaborated with The European Society for Blood and Marrow Transplantation (EBMT) to study the long-term outcomes of patients treated with Yescarta®. EBMT will primarily manage the registry follow-up, and such collaborations may be another cost-saving approach in some cases.
There are a few additional things to note regarding these costs:
- It is important to choose sites for registries carefully, ensuring they will be able to recruit a sufficient number of patients, given the high set-up and maintenance costs. A site that supports 100 patients over a period of time will be far more cost-effective than one that supports five.
- If a national registry already exists, then costs may be lower. However, the registry set-up must be fit for the purpose.
- Set-up costs per site are likely to drop as additional sites are established within a country. This is because materials and training programs can be repurposed.
- Costs vary by country, tending to be higher in the US and northern Europe vs. southern Europe and other regions.
- Another cost driver is the complexity of the monitoring schedule, which must be negotiated with national regulatory bodies.
As time goes on, it’s likely that the costs to set-up and run registries will drop. The increasing use of automation tools, the potential for patient self-reporting, and the expansion and/or enhancement of national and international registries should help push costs downward.
Coming Next
When developing commercial strategy for a gene therapy, planners must consider a wide range of complexities, including patient needs, caregiver and provider perspectives, the competitive environment, access and reimbursement factors, supply chain components, and the cost drivers described above.
In part IV, the final installment in this series, we’ll help “bring it all together.” Our goal is to provide a framework that planners can use to help structure their thinking as they work to consider all of those inter-related elements during commercial planning.