Developing a commercial strategy for any biopharma therapy can be a complex, detail-oriented process. It requires the collection of relevant scientific and market data, analytical skill to make sense of that data and derive insights from it, and a sound decision-making framework upon which to build an effective commercial model and strategy. That complexity multiplies when dealing with gene therapies.
In recent years, as gene therapies have become more widespread across various therapeutic areas, commercial leaders have increasingly had to deal with that complexity. For some, it’s a new environment and a bit of a departure from their previous experiences.
This article is the first in a 4-part series focused on commercial strategy in this area. Our goal is to provide some guidance for planners and decision-makers as they prepare to commercialize new gene therapies. There’s no way that we can provide fully comprehensive guidance in a relatively short, 4-article series. However, we will do the following:
- Provide a brief introduction to gene therapy, highlighting the differences between in vivo and ex vivo gene therapies from the patient’s point of view and other perspectives.
- Outline key supply chain considerations for gene therapies—which are critical to therapeutic and market success—and provide some high-level insights for decision-makers.
- Describe the primary cost drivers—and their impacts—to enable decision-makers to more effectively account for them during commercial planning.
- Offer a perspective on how decision-makers can structure their thinking when it comes to commercial strategy development.
Let’s get started!
Gene Therapy: A Brief Introduction
A gene therapy treats a genetic or inherited disease by replacing, deactivating, modifying, or introducing genes (with the help of a vector) in targeted cells. It alters a specific part of a patient’s genetic makeup to address a malfunction or mutation.
Gene therapies can be broadly organized into two groups, in vivo and ex vivo. Below, we provide a little more detail on the differences between these two types.
It’s important to note that there is some overlap between cell therapies and gene therapies. Therapies that involve genetically altering cells and then inserting them into the patient can be considered both cell and gene therapies.
In Vivo Therapies
This type of therapy involves directly delivering genetic material into a patient’s body, either systemically via intravenous infusion or locally to a specific organ or tissue. They are often used to treat disorders associated with the eye, brain, liver, skeletal muscles, or other organs.
The genetic material gets to the targeted cells using a vector. Vectors can be organized into two main groups: viral (most common for in vivo therapies) and non-viral.
Viral vectors are derived from viruses but they are incapable of causing infection. Rather, they act like a “delivery truck,” carrying the genetic material where it needs to go.
Non-viral vectors are more common with ex vivo therapies and can take different forms. For example, the genetic material can be introduced to a cell mechanically (using a needle), via chemical means, or via electroporation, which uses an electrical field to increase the permeability of a cell membrane.
In vivo therapies generally involve less complex manufacturing processes and streamlined treatment administration. From the patient’s point of view, the treatment is typically simpler than with ex vivo models, and the initial recovery post-treatment is shorter (often a week or less).
Figure 1 – In vivo models typically involve less complex production, streamlined treatment, and faster recovery times.
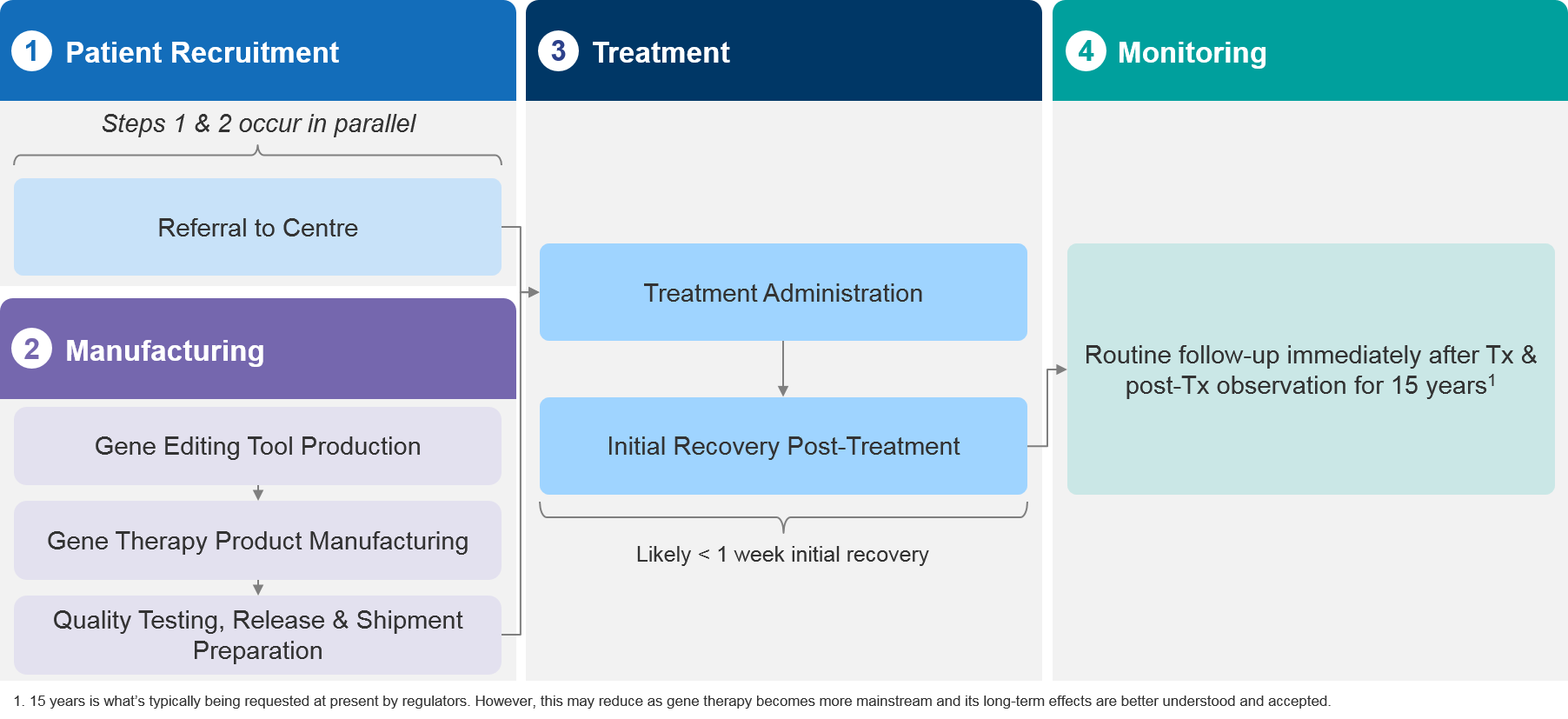
Ex Vivo Therapies
With ex vivo gene therapies, cells are collected from the patient, functional genetic material is then added to the cells using a vector, and the cells are then reintroduced into the patient. The modified cells then replace target cells with the missing or malfunctioning gene.
Ex vivo therapies are most often applied to stem cells, B-cells, T-cells, and NK (natural killer) cells. They can be used to treat blood diseases, neurological diseases, and other genetic diseases in organs or systems that blood cells can reach.
Ex vivo models are usually more complex than in vivo. They involve lengthy, custom manufacturing processes and longer administration and treatment cycles. From the patient’s point of view, they must usually endure a multi-step process that involves cell extraction, conditioning and pre-treatment, cell re-introduction and engraftment, and post-treatment recovery. The process can take well over a month.
Figure 2 – Ex vivo models are more complex, involving a bespoke manufacturing process, treatment preparation, and lengthy initial recovery process.
Key Factors Affecting Commercial Strategy
When developing a commercial strategy for a gene therapy, there are a number of factors that decision makers need to take into account. These include:
- The healthcare infrastructure in the market(s) in question
- The patient experience
- Access, pricing, and reimbursement
- Supply chain requirements
- Cost drivers
For any given therapy, the circumstances surrounding each of these factors will impact a company’s decision-making. Below, we describe factors 1-3 at a high level. In future installments in this series, we’ll tackle supply chain and cost drivers individually. In our final installment, we’ll tie it all together to offer some guidance that decision-makers can use as a tool for strategy development.
Market Infrastructure
Ex Vivo Therapies
Not all markets are the same, and the infrastructure available in a given area will affect planning efforts. Unsurprisingly, infrastructure challenges can be more acute when dealing with complex ex vivo therapies. Autologous ex vivo therapies typically require specialized apheresis centers for collecting cells from patients, which in turn require a relatively sophisticated healthcare market infrastructure. That doesn’t exist everywhere.
In such markets it might make sense for the company to qualify apheresis centers for the collection of specific cells required for the therapy. However, that can be a costly and time consuming endeavor, especially when one considers the investments required to train, qualify, maintain, and support them.
The expense may be worth it, though, if the company has a large ex vivo pipeline or if the market has a large potential patient population and a reasonable expectation of rapid and substantial uptake. In some cases, a company might be able to leverage existing centers that other companies have established. There will always be a level of additional training and qualification that’s required, but as gene therapy becomes more mainstream, newer companies should see more opportunities to piggy-back off of the pioneers in some markets.
Before deciding to qualify its own centers, a company would need to do a robust analysis and build the business case for it. If it’s not practical to qualify apheresis centers, then the company would need to leverage an out-of-country model in which patients must travel from their homes to a place that has the infrastructure needed. In that situation, the company must consider the additional burden that the travel and its associated costs (if not covered by payers) may place on patients and their caregivers.
Cell transduction is another part of the process, during which genetic material is introduced into the cells using a vector. Sending the blood / cells to be transduced (usually in a central facility abroad) comes with a lot of logistical limitations and handling requirements that must be taken into account. We’ll provide more on this in the next article.
Another key stage is therapy administration, which involves transfusing modified cells back into the patient. This takes place at a transfusion center that may or may not be the same location as the apheresis center. These facilities are specialized and will need to be accredited by JACIE[1] and/or other relevant organizations to conduct stem cell transplants.
Depending on the disease in question, the capacity at the transfusion centers can become a rate-limiting factor. For example, if it’s not a life-threatening disease, then a lot of beds might be reserved for patients who are undergoing cell transplant for deadlier diseases, which could mean a capacity issue for your therapy.
Or, if there is a lot of gene therapy competition in the market, a company may not be able to secure enough beds to realize a high throughput. Improving the myeloablation / conditioning regimen for patients can reduce the hospitalization time and hence, increase capacity. In any case, a company must understand how these dynamics could affect its therapy and the commercial model used to deploy it.
In Vivo Therapies
In vivo therapies are easier to apply in less advanced markets, as apheresis centers are not required. In addition, the transfusion centers do not need to be quite as specialized, so they are easier to find or establish.
Because there’s no myeloablation / conditioning or engraftment, patients may spend less time in transfusion centers, thereby increasing throughput and capacity vs. the ex vivo approach, so the whole process is easier to implement and scale. While in vivo therapies may be less complex than ex vivo, they aren’t exactly simple, and carry their own logistical and supply chain considerations. We’ll address those in part II.
Patient Experience
Throughout all of this, it’s critical to keep the patient’s experience and perspective in mind. We’ve already outlined why ex vivo therapies are typically more challenging for patients. More steps, time, inconvenience, and discomfort are involved.
There are other considerations, too. Because of the conditioning that is required, ex vivo therapies may be contraindicated for certain older or more frail patient groups. This reduces the eligible patient population vs. in vivo therapies unless conditioning regimens improve. Myeloablative therapies can also carry the risk of infertility, which can be a major concern for both men and women.
In addition, patients traveling abroad for apheresis or any other step (because they don’t have that infrastructure in their home country) face logistical and potential out-of-pocket cost challenges. Those challenges also apply if follow-up has to happen abroad.
Patient skepticism may also be a factor in some markets. Gene therapy is still in its early days, and some patients opt to take a “wait and see” approach, letting others try the therapy first before they try it for themselves. These patients are concerned about the potential short- and long-term consequences of new therapies, so they wait. This can obviously limit uptake.
During its planning efforts, a company must understand how patients will experience the therapy, as well as the barriers they may face. The commercial strategy must find a way to effectively remove barriers or at least make them more manageable, thus maximizing uptake and the number of patients who can get the help they need.
Access, Pricing, and Reimbursement
It’s no surprise that gene therapies are expensive. This can make securing the desired levels of access and reimbursement a challenge. In some cases, these therapies can represent a very large up-front cost with no guarantee of durable success due to their relative novelty and the fact that experience with them is limited. If a patient has to be sent abroad for apheresis—or any other step(s)—because the necessary infrastructure doesn’t exist in-country, then the costs for payers increase further.
Assuming a patient can get treatment, will the effect work? Will it last a lifetime or will the patient need to undergo it again? Questions like these have moved some biopharma companies, such as bluebird bio, to establish payment plans that are based on outcomes, where a payer’s outlay is tied directly to results and limited if the therapy fails to perform at a defined level.
Although in vivo therapies are typically easier to manufacture and administer than ex vivo therapies, they present similar challenges from a reimbursement standpoint. Payers must evaluate whether to reimburse these expensive therapies based on small studies and limited real world evidence.
Overall, the gene therapy market is in an early stage and the optimum payment and reimbursement approaches are still being sorted out. In some countries, biopharma companies may need to be pioneers working with payers to establish the right value assessment frameworks and payment models. Any commercial strategy for a gene therapy should incorporate a well-supported pricing and market access strategy, along with a healthy dose of innovative thinking.
Coming Next
In part II, we will address the key supply chain and logistics considerations that relate to gene therapies. As we’ve touched on already, gene therapies can have very complex manufacturing, distribution, handling, and administration requirements, which relate closely to the complex patient journeys associated with them. We will explore these requirements, highlighting key areas that decision-makers must deal with as they develop commercial models and strategies.
Notes:
[1] JACIE is The Joint Accreditation Committee ISCT (International Society for Cell & Gene Therapy) – Europe & EBMT (European Society for Blood and Marrow Transplantation). It is “Europe’s only official accreditation body in the field of haematopoietic stem cell transplantation (HSCT) and cellular therapy. It promotes high-quality patient care and medical and laboratory practice through a profession-led, voluntary accreditation scheme.” Source: https://www.ebmt.org/jacie-accreditation, accessed Aug. 8, 2022.