
As we’ve written many times before, Europe offers significant business opportunities for biopharmaceutical companies seeking to expand. Collectively, European countries represent the world’s second largest market for branded pharmaceuticals, just behind the United States.
With more than 500 million people and $140 billion in annual branded pharmaceutical sales, Europe can be highly attractive. It offers the potential for substantial revenue growth and increased organizational value. In addition, Europe can serve as a springboard to other regions of the world. For example, US-based companies that successfully enter Europe can leverage their positions there to access Asian, African, and Middle Eastern markets.
However, entering Europe successfully is not easy or simple. Its diverse markets, variable labor laws and tax structures, complex distribution requirements, differing payer environments, and multiple entry pathways can easily confuse new entrants…and confusion can lead to mistakes.
12 Pitfalls in Europe
When a growing company makes its move into Europe, it can face a range of potential pitfalls. In this series of articles, we will identify 12 of the most common pitfalls that emerging biopharma companies need to watch for as they enter European markets.
In each article, we will identify a pitfall, describe why it’s important, and offer some tips for avoiding it. In our experience, the 12 most common ones are:
- Not knowing what it takes to “go it alone” across Europe
- Not realizing Europe may have its own clinical endpoints in mind
- Missing the opportunity of conditional marketing authorization
- Believing EMA marketing authorization = patient access
- Thinking your list price won’t cross borders
- Missing the mark with local health technology assessment (HTA)
- Not making the right European connections
- Failing to realize that EAPs can boost…or bite
- Missing a promotable label
- Building like big pharma when you’re not
- Using individual preferences vs. analysis to select HQ location
- Caring too late about your infrastructure
Without further delay, let’s take a look at our first pitfall.
Pitfall #1 – Not knowing what it takes to “go it alone” across Europe
Why It’s Important
In this context, “going it alone” means establishing a physical presence in a country, becoming the marketing authorisation holder (MAH), fielding management and commercial teams, and commercializing a product without relying on licensing agreements, co-promotion partners, or similar arrangements. Doing all that requires substantial investment and risk, though its rewards can be great.
No emerging company should fool itself into thinking that it can take on all of Europe with a “go it alone” approach. The required investments and risks will typically outweigh its capabilities, and companies that try to go it alone in all of their targeted European markets risk over-extending themselves. At best, that can result in a sub-optimal outcome. At worst, it can result in total failure.
Avoiding It
In most cases 80% of the market value in Europe is within the EU4 (France, Germany, Spain, and Italy) plus the UK. So, for most companies, entering Europe becomes a process of strategically “picking their battles” as they must decide which markets to enter, how best to enter each, and whether those decisions need to vary by product.
To make these decisions on a market by market (and/or product by product) basis, companies must balance their desired level of control—and the value they hope to realize—against the associated risk and financial commitment. Where the optimum combination lies for a given company can help determine which entry option it chooses for a specific country or region.
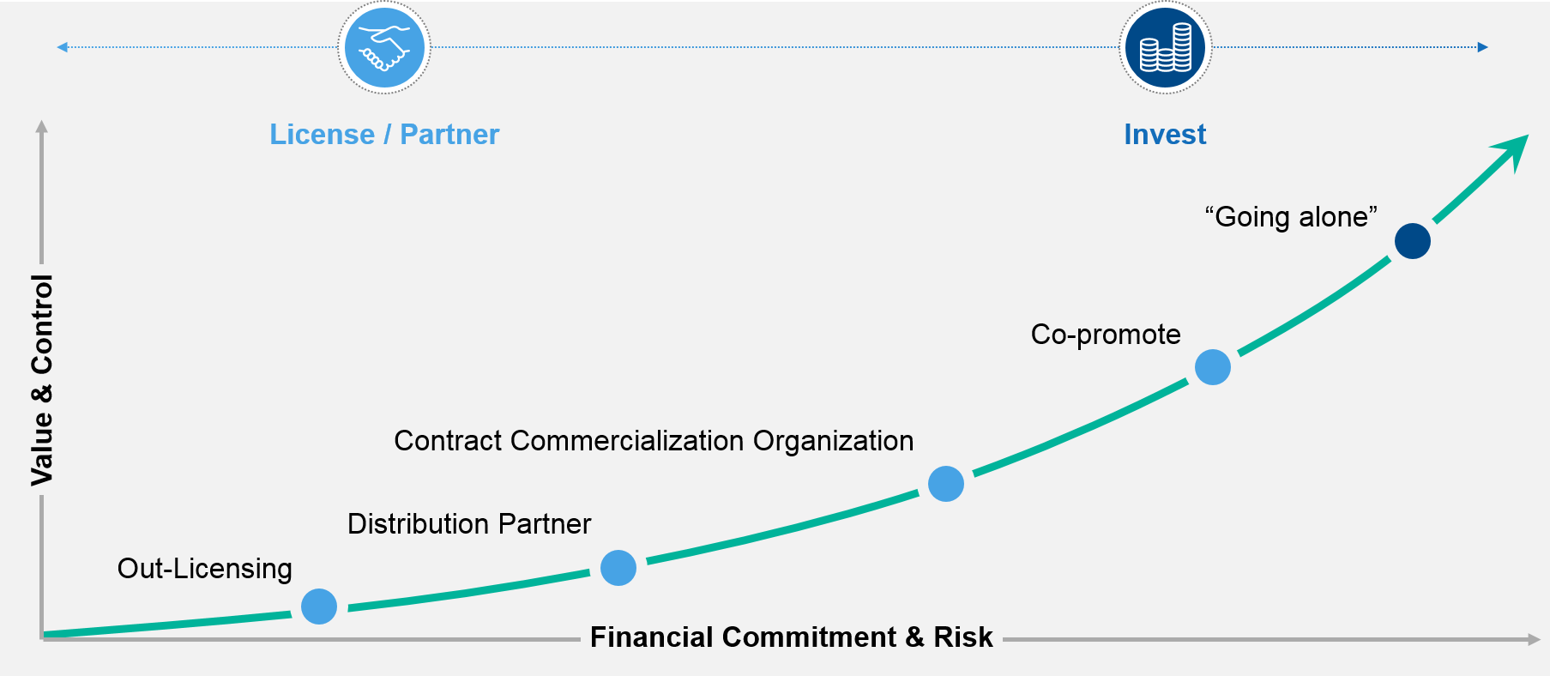
In the end, a company will typically enter Europe via a combined approach that involves going it alone in priority markets and leveraging partners for others. For example, a company might enter the major European markets on its own, but then rely on licensing agreements or other partnering deals to enter other (smaller) western European nations, key eastern European markets, the Baltic states, and so on. Also, a company may choose to not build all capabilities in-house from the start and balance 3rd party involvement versus internal FTE ratios according to decision gates as the program de-risks.
As the company matures, the approach may change. Typically, an emerging company will start with a mix of entry options and move towards a more “go-it-alone” approach as it grows. And, as companies become established multinational organizations like Amgen, Alexion and Biogen, the reverse can be observed, i.e., “off-loading” assets to distribution partners in some regions.
The key to avoid pitfall #1 is to carefully weigh your goals for each target market against the risks and investments required. By using a deliberate and analytical approach, you can find the optimum balance—and the right entry strategy—for each one.
Coming Next
In the next article, we’ll address pitfall #2: Not realizing Europe may have its own clinical endpoints in mind. As you’ll see, this article will go beyond just clinical endpoints and include many aspects of a product’s data package. The many different jurisdictions in Europe often have different interests and priorities regarding the data they need to see, and it’s critical to understand that.






