
Psychedelics and other traditionally taboo substances appear to be undergoing a bit of a “renaissance” in central nervous system (CNS) therapeutics these days. These types of substances have a long and storied history all around the world. Now, science is getting serious about exploring ways to use them to treat a range of diseases and disorders. In this article, we take a look at some of the substances that are getting the most attention, as well as the companies and investors that are driving development in this space.
Ups and Downs Throughout History
Hallucinogenic cactuses, plants, and mushrooms have been used to induce altered states of consciousness in healing rituals and religious ceremonies for many centuries.[i] Some examples include balché (a mixture of honey and extracts of Lonchocarpus), peyote (which contains mescaline), and seed of ololiuhqui (which contains lysergic acid amide, a precursor to LSD).
Cannabis Sativa is another example. It’s one of the oldest cultivated plants, with evidence of cultivation going back almost 5,000 years to northeast China. It was used medicinally and as a source of food and fiber. As a drug, cannabis has been recognized as a sacred plant in Hindu scripture dating back more than 3,000 years.[ii]
Fast-forwarding to the 20th century, research using these hallucinogens and cannabis was thriving in the West during the 1940s and 1950s. These efforts were generating a large body of initial data regarding the potential clinical benefits of these substances.
During the 1960s, however, research cooled considerably. In the US, the federal government classified many of these drugs as schedule 1 restricted substances which require a special license to possess. This made research difficult and helped push these substances into the shadows for decades.[iii]
Over the past 20 years, things have been changing. The medical cannabis movement has expanded dramatically. Its growth has also focused attention on other taboo substances, and society has started to reconsider the potential for these drugs to be used therapeutically. Now, the market for treatments using psychedelic active ingredients is projected to reach $6.85 billion by 2027 with a CAGR of 16.3% over the next 7 years (not including cannabinoids).[iv]
A lot is changing in the field of psychedelics. In January 2021, the first psychedelics exchange traded fund (ETF), Horizons Psychedelic Stock Index (PSYK), debuted on the NEO exchange.[v] There is also a movement to decriminalize psilocybin mushrooms in several cities and the substance has been legalized for use in therapy in Oregon.[vi] In addition, Field Trip Health has established the world’s first legal psilocybin research and cultivation facility in Jamaica to determine how psilocybin-producing mushrooms can be used in medicine.[vii]
Examples of Taboo Substances Being Studied Today
Psilocybin
Psilocybin was first isolated by Albert Hofmann in 1957 from Psilocybe mexicana, a psychedelic mushroom. Psilocybin is a prodrug of psilocin, a non-selective serotonin 2A receptor (5-HT2AR) agonist. It is considered, along with LSD and N, N dimethyltryptamine (DMT), to be a classic hallucinogen.[viii]
It was Initially sold in the 1960s under the name Indocybin by Sandoz. Currently, it is being studied as a potential treatment for depression, anxiety disorders, obsessive-compulsive disorder (OCD), and for alcohol and tobacco use disorder.[ix]
LSD
Lysergic acid diethylamide (LSD) was synthesized in 1938 and its psychoactive effects were discovered in 1943. In the 1950s LSD (Delysid by Sandoz) was introduced into psychotherapeutic treatments. Throughout the 1950s and 1960s, it was used as an experimental drug in psychiatric research to produce “experimental psychosis” for psycholytic and psychedelic therapy.[x]
Current interest in LSD is as a possible treatment for cluster headache, as a psychotherapeutic treatment for the terminally ill, as a treatment for alcohol use disorder, and for psychological disorders including anxiety and depression.
Ketamine
Ketamine was first used in humans in 1965 as an anesthetic. It is a non-selective NMDAR antagonist having an affinity for different NMDA receptor types.
Ketamine administration has long been known to mediate a wide variety of pharmacological effects including dissociation, analgesia, sedation, catalepsy, and bronchodilation. Recent research has uncovered multiple novel potential uses for this drug including neuroprotection, combatting inflammation and tumors, treatment of depression, seizures, chronic pain, and headache.[xi] However, its side-effect profile and potential for abuse—like many taboo substances—cannot be ignored. Currently, there are more than 800 clinical trials exploring the non-anesthetic use of ketamine listed on clinicatrials.gov.
Phytocannabinoids
The first reported use of cannabis is in a Chinese pharmacopeia that dates back to 2,600 BC. In the 1960s, delta9-THC was isolated and synthesized and found to be the primary psychoactive constituent of marijuana.
Phytocannabinoids—Cannabidiol (CBD) and delta-9-tetrahydrocannabinol (delta-9THC)—interact uniquely with the endocannabinoid system (ECS). Through direct and indirect actions, intrinsic endocannabinoids and plant-based phytocannabinoids modulate and influence a variety of physiological systems influenced by the ECS.
Neurological applications have been a major focus of renewed research using medical marijuana and phytocannabinoid extracts. Potential uses for phytocannabinoids include treatment of Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, neuropathic pain, seizures from genetic epilepsy disorders, mood disorders, anxiety, depression, addiction, post-traumatic stress disorder (PTSD), and as an adjunctive treatment for malignant brain tumors.[xii]
Current and Emerging Players
Currently, there are a few marketed drugs with taboo active ingredients and some emerging market leaders focused on the development of taboo treatments with several assets in clinical trials. Below, we provide a brief overview.
Key Marketed ‘Taboo’ Drugs
Janssen
Janssen’s Spravato® (esketamine) is an anti-depressant intranasal spray using S-ketamine, the S-enantiomer of ketamine. Approved by the US FDA in 2019 for treatment-resistant depression, it is indicated for use in conjunction with an oral antidepressant. Since 2019, it has also been approved to treat depressive symptoms in
- patients with major depressive disorder (MDD) with acute suicidal ideation or behavior (2020)
- psychiatric emergency for patients with MDD (2021)
Global sales are expected to grow to $882 million in 2026, with further growth to follow.
Jazz Pharmaceuticals
In February of 2021, Jazz Pharmaceuticals announced the acquisition of GW Pharmaceuticals, Plc for $7.2 billion.[xiii] GW’s lead product, Epidiolex® was the first plant-derived cannabinoid medicine approved by the US FDA (2018).[xiv] It was approved to treat patients one-year and older for seizures associated with Lennox-Gastaut syndrome (LGS), tuberous sclerosis complex, and Dravet syndrome.
Epidiolex was also approved in Europe under the name Epidyolex in September of 2019. The therapy’s global sales are $510 million and growing rapidly (CAGR of more than 27%). Global sales are expected to surpass $1.6 billion in 2026.
During 2020, GW also started a phase 3 clinical trial for another cannabinoid drug, nabiximols, for spasticity in multiple sclerosis (MS). Four additional trials will begin in 2021 with some results expected in mid-year. Nabiximols, marketed as Sativex®, is already approved for use in over 25 countries outside the US (including the UK) to treat MS spasticity.
GW is also investigating CBDV, a non-psychoactive cannabinoid, in epilepsy and autism spectrum disorder (ASD). The therapy is currently in phase II clinical trials.
Key Companies with Treatments in the Clinical Development Pipeline
COMPASS Pathways
After receiving a breakthrough designation from the US FDA in 2018, COMPASS Pathways is conducting the world’s first large-scale psilocybin therapy clinical trial.[xv] The trial involves 20 sites across 9 countries in Europe and North America. It is studying the use of psilocybin for treatment-resistant depression. Multiple investors have expressed interest in backing COMPASS pathways including Otsuka Pharmaceuticals and ATAI Life sciences AG.
Mind Medicine
Mind Medicine is a front-runner in this space with novel psychedelic therapies moving through clinical trials. It is currently running trials investigating:
- Micro-dosing LSD for attention deficit / hyperactivity disorder (ADHD)
- 18-MC, an ibogaine-derived medication to treat addiction, with lower risk of heart attack than ibogaine
- MDMA (“Ecstasy” or “Molly”) combined with LSD to reduce the side-effects of LSD, potentially making it more suitable for therapeutic uses[xvi]
- LSD experiential therapy for anxiety disorders, launched as Project Lucy and currently in phase 2b
Seelos Therapeutics
Seelos Therapeutics is a clinical-stage biopharmaceutical company focused on the development of therapies for CNS and rare diseases. It is currently testing SLS-002, an intranasal racemic ketamine for acute suicidal ideation and behavior (ASIB) in patients with MDD and PTSD.
The trials involve a two-part proof-of-concept study with part one being open-label with 16 patients and part two being a double-blind placebo-controlled study with approximately 120 patients randomized 1:1. Part 1 in ASIB (MDD) was completed in March of 2021 and is expected to release key open-label data in the second quarter.[xvii]
Incannex Healthcare
Incannex is focused on developing cannabinoid medicines. The company currently has research in four therapeutic areas:
- IHL-42X in obstructive sleep apnea (phase 2b)
- IHL-216A in traumatic brain injury (preclinical)
- IHL-675A in sepsis associated acute respiratory distress syndrome with an oral medication of hydroxychloroquine (HCQ) and cannabidiol (CBD) (preclinical)
- IHL-493C in temporomandibular joint dysfunction (preclinical)
In December of 2020, Incannex also partnered with Monash University to conduct a phase 2 randomized double-blind clinical trial using psilocybin-assisted psychotherapy to treat generalized anxiety disorder[xviii]
Table 1: Key Current and Emerging Players in “Taboo” Medicines
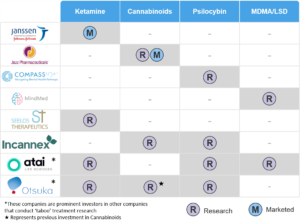
The companies listed above are not the only players in this space, and we can see Table 1 expanding rapidly as attention on “taboo” substances intensifies. For example, another emerging firm in the space is Delix Therapeutics, a company developing “psychoplastogens” which are similar to psychedelics, but without the hallucinogenic properties.[xix]
Our Perspective
The “taboo” market is definitely becoming a lot less taboo. It is healthy and growing with increased backing from a combination of famous and prominent investors, investments funds, and pharmaceutical companies. Below, we highlight some of the people and companies that are funneling investments into this type of research.
Prominent investors
Peter Thiel, German-American entrepreneur and venture capitalist—and co-founder of PayPal—has joined a syndicate putting $125 million into ATAI Life Sciences. ATAI is a Berlin-based biotech firm with a portfolio of psychedelic drugs targeting depression, anxiety and addiction. ATAI has been busy:
- In January of 2019, ATAI acquired Perception Neuroscience. Its lead product is an NMDA antagonist, PCN-101 (R-ketamine) currently under development for treatment-resistant depression (TRD). A phase I clinical trial showed positive results and a Phase IIa proof-of-concept study is anticipated to begin in the second quarter of this year. Related to this, in March of this year, Perception Neuroscience announced a collaboration with Otsuka to develop PCN-101 in Japan for treatment of depression.[xx]
- In January of 2020, ATAI formed a joint venture with DemeRx, a clinical stage pharmaceutical company focused on developing Noribogaine (DMX-NB1) in Phase II and Ibogaine (DMX-IB1) in Phase I as oral, non-addicting treatments for opioid dependence.
- In August of 2020, ATAI launched EmpathBio, a subsidiary focused on developing MDMA derivatives for the treatment of PTSD and other indications.
- In January of 2021, ATAI started a partnership with Mass General Hospital. ATAI is the first partner in Mass General’s Center for Neuroscience of Psychedelics, which is working to understand the mechanisms that underlie the therapeutic effect of psychedelic agents.
- In March of this year, ATAI recently closed a $157 million series D round, completing a four-month span in which it raised nearly $300 million.
- Interestingly, ATAI has also expressed a keen interest in combining digital therapeutics with psychedelics. It has launched IntroSpect to focus on that area.[xxi]
Investment Funds
Neo Kuma Ventures, a London-based venture capital firm, has established Britain’s first psychedelic healthcare fund. Dedicated solely to psychedelic healthcare, the fund launched in December of 2020.
The fund has already attracted millions of pounds in investment and will continue to draw venture capital through the first half of 2021. Its investment thesis is focused on “the most exciting, high quality, and scientifically sound players in the industry.”
The Noetic Fund, launched by Grey House Partners GP, Inc., is also investing in this space. It focuses on “emerging and early-stage wellness, therapeutic and pharmaceutical companies around the globe.”[xxii]
Another interesting player is What If Ventures, a venture capital firm formed in early 2020 that focuses on mental health and addiction recovery. Its portfolio includes various firms in the “taboo” space. According to Graham Smith, a Partner with What If Ventures, “Five of our last 13 deals have been in the psychedelic space, including ATAI. We expect to complete several more raises for psychedelic companies this year. We are really excited about the potential in the space.”[xxiii]
Large Pharmaceutical Companies
Large pharmaceutical companies are also getting into the mix. For example, Otsuka has backed COMPASS Pathways, mentioned previously. On September 18, 2020, COMPASS had its initial public offering (IPO). Its initial valuation was expected to be around $544 million, but its market capitalization in now $1.91 billion.[xxiv]
Moving forward, several other leading biopharma companies may be interested in this space:
- Otsuka: Otsuka already has a strong focus on CNS treatments and, as mentioned above, has already backed COMPASS. Look for more potential involvement and investment from Otsuka in this space.
- Biogen: Assuming aducanumab fails, Biogen may want to go into this space next due to its CNS focus.
- Takeda/Shire: Takeda/Shire has a strong base in CNS research and is a leader in ADHD treatments.
- Janssen: Janssen is already a player in the market with Spravato (esketamine) for MDD.
- AbbVie/Allergan: AbbVie/Allergan has the rights to a novel psychedelic-based treatment for MDD that failed to meet its primary endpoint. However, the company may still be interested in the space moving forward.
Clearly, there is a lot of renewed focus on studying the use of “taboo” treatments for therapeutic purposes. This will be an interesting space to watch as more of the research is completed and data is read out. Regardless, we believe that these treatments are here to stay given the CNS need and the early success of drugs like Spravato. There is a great deal of promise in “taboo” treatments, and we expect to see these drugs to have a veritable impact in treating CNS diseases as this market matures.
References
[i] Carod-Artal, F.J. (2015) Hallucinogenic drugs in pre-Columbian Mesoamerican cultures, Neurologia, vol. 30 (issue 1), pp. 42-49, https://pubmed.ncbi.nlm.nih.gov/21893367/
[ii] Garcia-Romeu, Albert, et al, (2016) Clinical Applications of Hallucinogens: A Review, Experimental and Clinical Psychopharmacology, vol. 24 (issue 4), pp. 229-268, https://doi.apa.org/doiLanding?doi=10.1037%2Fpha0000084
[iii] Nutt, David, (2015) Illegal Drugs Laws: Clearing a 50-Year-Old Obstacle to Research, PLOS Biology, https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.1002047
[iv]U.S. Psychedelic Drugs Market – Industry Trends and Forecast to 2027, Data Bridge Market Research, June, 2020, https://www.databridgemarketresearch.com/reports/us-psychedelic-drugs-market
[v] Ponieman, Natan, First Psychedelics ETF Debuts On NEO Exchange, MarketWatch.com, Jan. 27, 2021, https://www.marketwatch.com/story/first-psychedelics-etf-debuts-on-neo-exchange-2021-01-27?mod=investing
[vi] Roberts, Chris, Oregon Legalizes Psilocybin Mushrooms and Decriminalizes All Drugs, Forbes, Nov. 4, 2020, https://www.forbes.com/sites/chrisroberts/2020/11/04/oregon-legalizes-psilocybin-mushrooms-and-decriminalizes-all-drugs/?sh=4da17f774b51
[vii] Dutton, Gail, Field Trip Health’s New Psilocybin Research Facility Probes the Secrets of Shrooms, BioSpace, Feb. 26, 2021, https://www.biospace.com/article/field-trip-health-s-new-psilocybin-research-facility-probes-the-secrets-of-shrooms/?utm_campaign=GenePool&utm_medium=email&_hsmi=113141700&_hsenc=p2ANqtz
[viii] Shore, Ron Joseph, et al (2019), Mapping Psilocybin-Assisted Therapies: A Scoping Review, https://www.medrxiv.org/content/10.1101/2019.12.04.19013896v3
[ix] Daniel, Jeremy and Haberman, Margaret (2017), Clinical potential of psilocybin as a treatment for mental health conditions, Mental Health Clinician, vol. 7 (issue 1), https://meridian.allenpress.com/mhc/article/7/1/24/127704/Clinical-potential-of-psilocybin-as-a-treatment
[x] Tassie, P., et al, (2008) The Pharmacology of Lysergic Acid Diethylamide: A Review, CNS Neuroscience and Therapeutics, https://onlinelibrary.wiley.com/doi/full/10.1111/j.1755-5949.2008.00059.x
[xi] Pribish, A, Wood, N, and Kalava, A (2020), A Review of Nonanesthetic Uses of Ketamine, Anesthesiology Research and Practice, https://pubmed.ncbi.nlm.nih.gov/32308676/
[xii] Joseph Maroon, Jeff Bost. Review of the neurological benefits of phytocannabinoids. 26-Apr-2018;9:91. Available from: https://surgicalneurologyint.com/surgicalint-articles/review-of-the-neurological-benefits-of-phytocannabinoids/
[xiii] Company Press Release, Jazz Pharmaceuticals, Jazz Pharmaceuticals to Acquire GW Pharmaceuticals plc, Creating an Innovative, High-Growth, Global Biopharma Leader, Feb. 3, 2021, https://investor.jazzpharma.com/news-releases/news-release-details/jazz-pharmaceuticals-acquire-gw-pharmaceuticals-plc-creating
[xiv] Press Release, US Food & Drug Administration, FDA Approves First Drug Comprised of an Active Ingredient Derived from Marijuana to Treat Rare, Severe Forms of Epilepsy, June 25, 2018, https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms
[xv] Company Press Release, COMPASS Pathways, COMPASS Pathways Receives FDA Breakthrough Therapy Designation for Psilocybin Therapy for Treatment-resistant Depression, Oct. 23, 2018, https://www.prnewswire.com/news-releases/compass-pathways-receives-fda-breakthrough-therapy-designation-for-psilocybin-therapy-for-treatment-resistant-depression-834088100.html
[xvi] Company Press Release, MindMed, MindMed Announces First-Ever Clinical Trial Combining MDMA and LSD, August 25, 2020, https://mindmed.co/news/press-release/mindmed-announces-first-ever-clinical-trial-combining-mdma-and-lsd/
[xvii] Company Press Release, Seelos Therapeutics, Seelos Therapeutics Announces Completion of Open-Label Patient Enrollment of Proof of Concept Study of SLS-002 (Intranasal Racemic Ketamine) for Acute Suicidal Ideation and Behavior in Patients with Major Depressive Disorder, March 5, 2021, https://www.prnewswire.com/news-releases/seelos-therapeutics-announces-completion-of-open-label-patient-enrollment-of-proof-of-concept-study-of-sls-002-intranasal-racemic-ketamine-for-acute-suicidal-ideation-and-behavior-in-patients-with-major-depressive-disorder-301241165.html
[xviii] Nicholas, Lorna, Incannex Healthcare and Monash University to undertake ‘world first’ trial using psilocybin mushrooms to treat anxiety, Small Caps, Dec. 8, 2020, https://smallcaps.com.au/incannex-healthcare-monash-university-trial-psilocybin-mushrooms-treat-anxiety/
[xix] Al Idrus, Amirah, Shire neuro head lands at Delix Therapeutics as CEO, Fierce Biotech, Mar. 18, 2021, https://www.fiercebiotech.com/biotech/shire-neuro-head-lands-at-delix-therapeutics-as-ceo
[xx] Company Press Release, ATAI Life Sciences, Perception Neuroscience And Otsuka Pharmaceutical Announce Collaboration On Development Of PCN-101 (R-ketamine) In Japan For Treatment-of Depressive Disorders, March 16, 2021, https://www.prnewswire.com/news-releases/perception-neuroscience-and-otsuka-pharmaceutical-announce-collaboration-on-development-of-pcn-101-r-ketamine-in-japan-for-treatment-of-depressive-disorders-301247872.html
[xxi] Corporate Website, accessed Mar. 17, 2021, https://www.atai.life/programs/introspect/
[xxii] Corporate Website, accessed Mar. 17, 2021, https://noeticfund.com/
[xxiii] Direct conversation with Graham Smith, Mar. 16, 2021, quoted with permission.
[xxiv] Peters, Bill, First Psychedelic Drug Stock Hits $1 Billion In Debut After Upsized IPO, Investor’s Business Daily, Sept. 18, 2020, https://www.investors.com/news/compass-pathways-sees-544-million-valuation-first-psychedelic-drugs-ipo/





