Over the past 5 years, several ground-breaking advancements in oncology have dramatically advanced the standard of care. These new classes of drugs include the PD(L)1 checkpoint inhibitors, the PARP inhibitors, and the EGFR resistance targeted drugs. Also included is a general trend towards more precise and targeted treatment decisions, driven by incorporating molecular tests and panels into the standard of care.
In parallel, we’re beginning to reap the benefits of “smarter” regimens which target underlying tumor biology via combinatorial or sequential approaches intended to boost efficacy and / or block mechanisms of resistance, for example
- BRAF/MEK combos in BRAF-mutated melanomas) that address BRAF-driven resistance to MEK inhibitors alone
- The emerging use of PARP inhibitors in the adjuvant setting of BRCA mutated tumors
Here, we review key advances in oncology from 2014 until mid-2019, highlighting common themes which will drive continued success. In future papers, we’ll discuss the organizational, regulatory, and commercial requirements that will enable pharmaceutical and diagnostic companies to succeed in this future environment.
Introduction to Key Trends
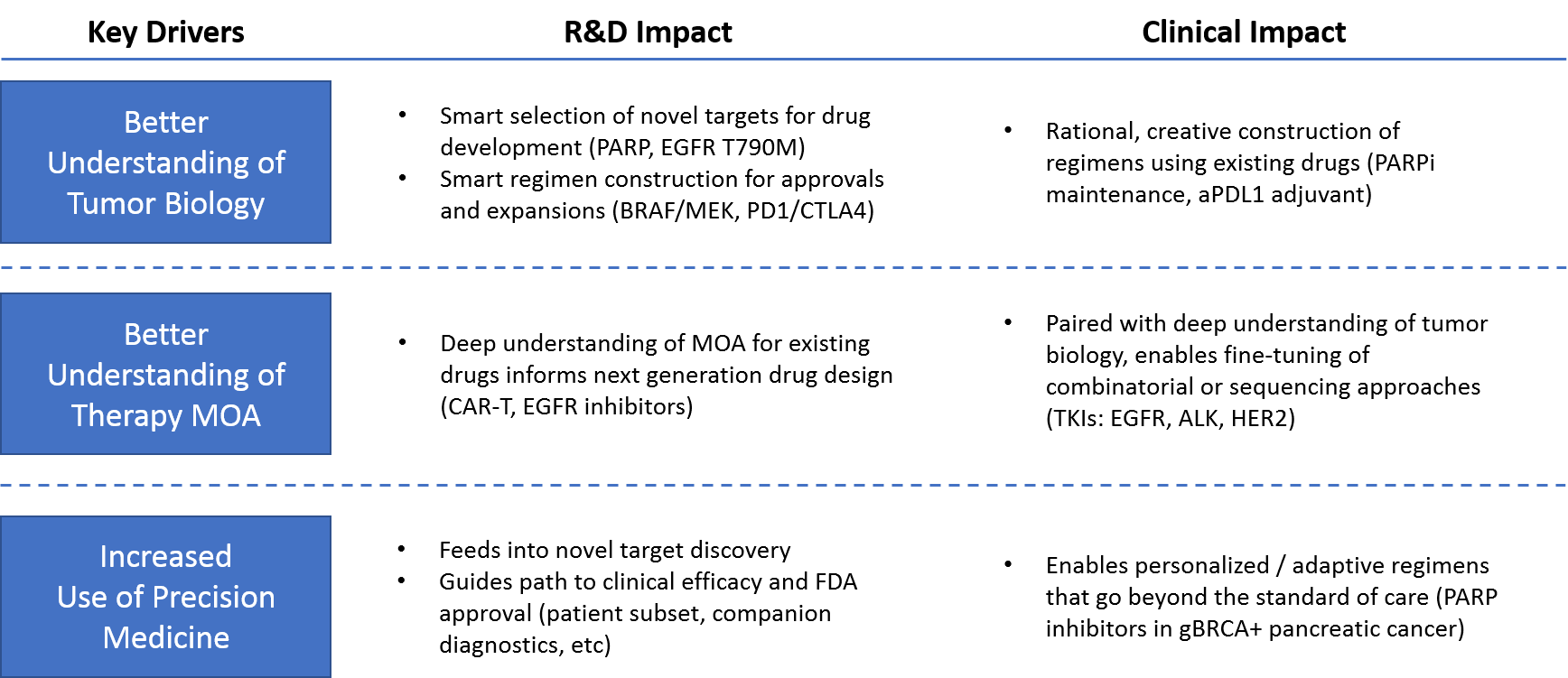
Over the last 5 years, we have enjoyed a positive feedback loop in which increasingly in-depth scientific understanding has informed research & development strategies and accelerated the clinical impact of emerging drugs and diagnostics. We see evidence of this in the:
- Launch of numerous novel targeted drugs (and classes of drugs)
- Expansion of coverage and initial FDA approvals of the broad panel diagnostic tests
- Increasing integration of both into precision medicine
These advances continue and accelerate, as learnings from these new products inform next generation product design and help improve regimen construction with the drugs we already have.
Recent scientific advances represent three key drivers of recent and ongoing success in oncology:
- A better understanding of tumor biology, which increases new drugs’ probability of success
- A better understanding of therapy MOA, which helps improve the way therapies and regimens are constructed and applied
- The increased use of precision medicine, which ensures that each patient receives the most appropriate regimen for his or her cancer
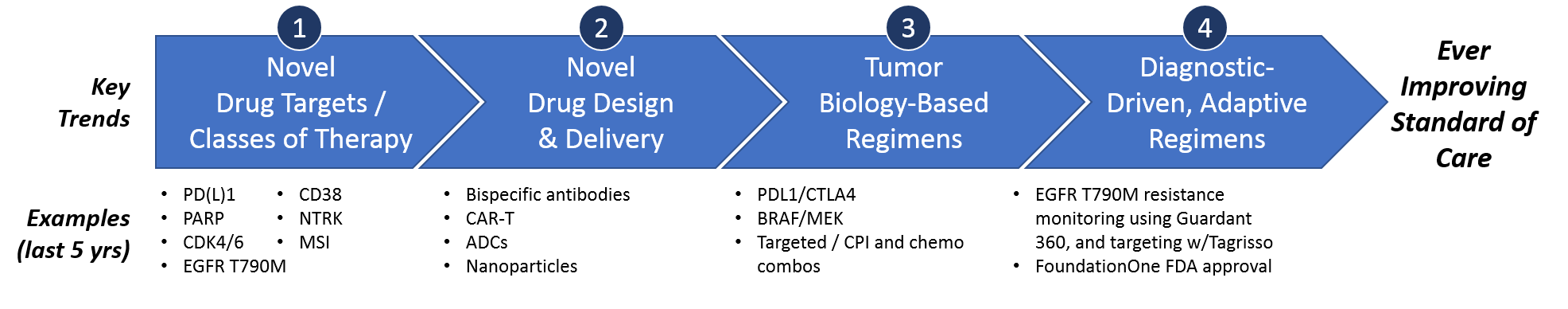
Over the past 5 years, these key drivers have delivered several impactful trends towards an ever-improving standard of care. Namely, these include novel targeted drugs, novel drug design approaches, tumor biology-based combinatorial or sequential regimens, and (most impactfully) truly personalized diagnostic-driven, adaptive regimens.
We have seen major successes in each of these categories, as we discuss in more detail below. In part II of this series, we also identify some remaining unmet needs and areas where these trends are likely to deliver further success over the next 5 years.
Trend 1 – Novel Drug Targets & Classes of Therapy
Since 2014, almost 60 new cancer drugs have launched across solid and liquid tumor types. Several have launched multiple follow-on indications, leading to ~90 labeled indications for these new drugs.
Of these new drugs, about half were aimed at only 17 novel targets. Some of these targets are very tumor type-specific, for example IDH1 and IDH2, which are frequently mutated in leukemias and gliomas, but only rarely in other cancers.
Other targets are quite broadly mis-regulated across numerous tumor types, for example BRCA (targeted by PARP inhibitors), CDK4/6, PI3K, NTRK, and most notably the PD1/PDL1 checkpoint inhibitor proteins, which are overexpressed in subsets of varying sizes across all tumor types. ROS1 is another interesting example, as the drug which targets it (crizotinib) was previously approved as an ALK inhibitor. However, this approval for a ROS1+ specific indication in NSCLC represents a novel target. This approval is a likely early indication of an emerging trend around better understanding and application of the protein kinase inhibitor class of drugs, which frequently have broad effect across multiple targets.
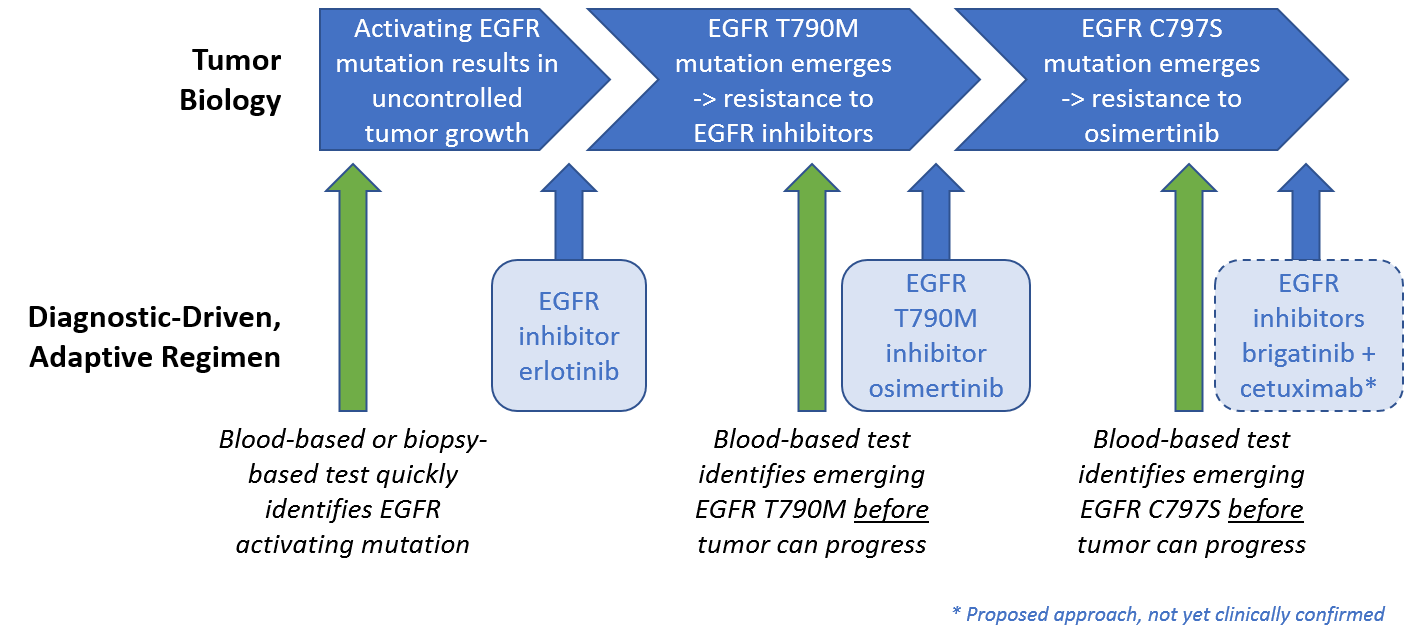
A third category of novel targets are those which were selected based on known mechanisms of resistance. Examples include EGFR T790M as a known mutation for EGFR inhibitor resistant NSCLC.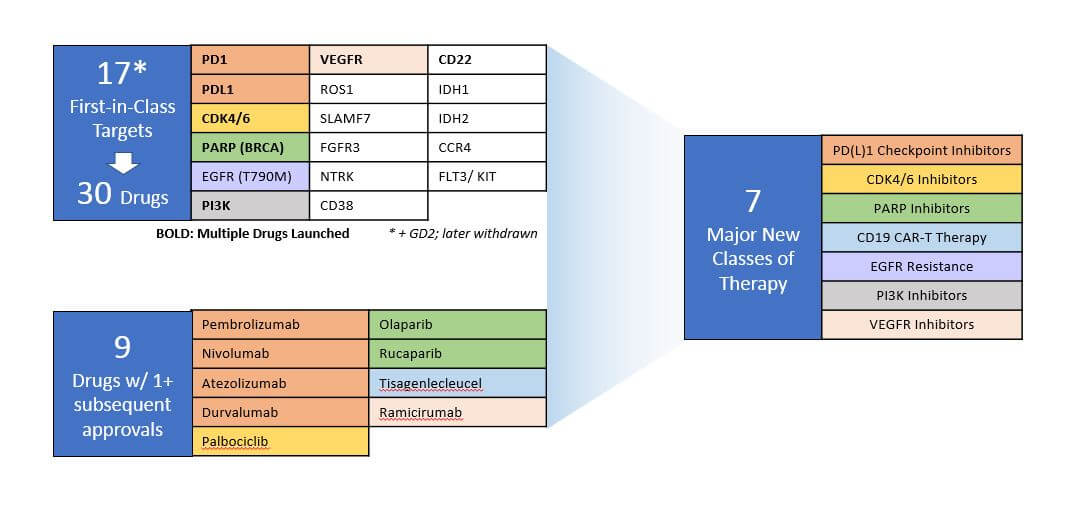 Since their initial launch, several of these drugs achieved subsequent approvals across additional indications and / or had follow-on approvals of other drugs aimed at that same target. Along with the CD19 CAR-T therapies, which represent a novel drug design but not a novel target, they represent the introduction of 7 new major classes of therapies with significant clinical impact and commercial success, many of them with broad efficacy across multiple cancer types.
Since their initial launch, several of these drugs achieved subsequent approvals across additional indications and / or had follow-on approvals of other drugs aimed at that same target. Along with the CD19 CAR-T therapies, which represent a novel drug design but not a novel target, they represent the introduction of 7 new major classes of therapies with significant clinical impact and commercial success, many of them with broad efficacy across multiple cancer types.
The PD1/PDL1 checkpoint inhibitors, in particular, have dramatically shifted treatment paradigms from the classic targeted approaches (a drug aimed at a particular oncogenic mutation) towards an immunotherapy-targeted approach. This approach seeks to activate and boost the immune system more generally to target and destroy tumor cells.
In leukemias, the CD19 CAR-T therapies also induce an immune response via a novel drug design that “forces” immune recognition of tumor-specific targets. They have shown curative benefit in some patients.
In the more classic targeted arena, drugs that target CDK4/6, PARP, and EGFR T790M have dramatically improved outcomes in specific settings. They have been incorporated into combinatorial and sequential regimens designed to outsmart known tumor biology.
PI3K inhibitors have great potential as the PI3K/AKT/mTOR pathway is very frequently mis-regulated in tumors. These drugs have had relatively limited success as monotherapies. However, now that they’ve launched and are available for clinical exploration, they’re showing promise as part of tumor biology-based regimens along with other targeted drugs.
Trend 2 – Novel Drug Design
Even with a drug that can effectively target a tumor-specific molecular alteration, it can be difficult to properly deliver it to the tumor cells so it can effectively treat the disease. In addition, it’s also important to minimize toxicity and the inadvertent killing of healthy cells. Fortunately, there have been major advances in drug design to boost the efficacy of existing targeted drugs and / or new drugs against known targets.
The common theme in drug design the past 5 years has been a modular design that physically attaches a cancer killing agent to a tumor targeting agent to more safely deliver an efficacious dose to the tumor with less impact on surrounding healthy tissues. This is typically done by leveraging naturally occurring biology (antibody-drug conjugates, engineered immune cells, or engineered viruses) or by attaching nanoparticles that result in better infiltration to the tumor. A related approach has been genetic engineering or modification of living immune cells or viruses to make them specifically target and destroy tumor cells.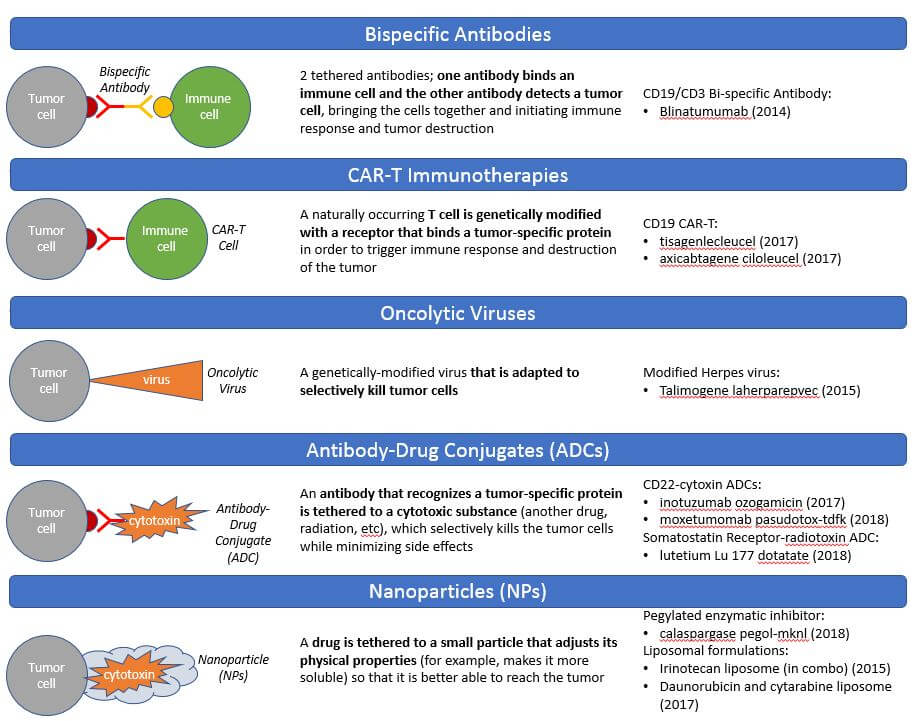 The CAR-T therapies are the breakout success in this arena, with curative efficacy in leukemias which express the CD19 target on their cell surfaces. While the first-generation CAR-T designs have so far not proved highly effective in solid tumors, we anticipate continued investment in next-generation designs that will hopefully translate this efficacy into solid tumors.
The CAR-T therapies are the breakout success in this arena, with curative efficacy in leukemias which express the CD19 target on their cell surfaces. While the first-generation CAR-T designs have so far not proved highly effective in solid tumors, we anticipate continued investment in next-generation designs that will hopefully translate this efficacy into solid tumors.
Trend 3 – Tumor Biology-Based Regimens
We have long known that many cancers cannot be completely destroyed by a single therapy alone, and instead require one or both of more complex regimen types designed to better address tumor biology:
- Combination regimens designed to target multiple aspects of known tumor biology and kill more of the tumor at once
- Sequential regimens designed to target known tumor biology with the goals of sensitizing a tumor to subsequent therapy, or preventing recurrences after a previous therapy (neo-adjuvant and adjuvant or maintenance therapies, respectively)
Over the past 5 years, we have seen significant advancements in both approaches, reflected both by standard of care as well as new drug approvals. In fact, a notable portion of the total novel drug approvals in the last 5 years were in combinations, i.e., the new drug was not approved to be used alone but instead as a regimen with chemo or with another targeted therapy.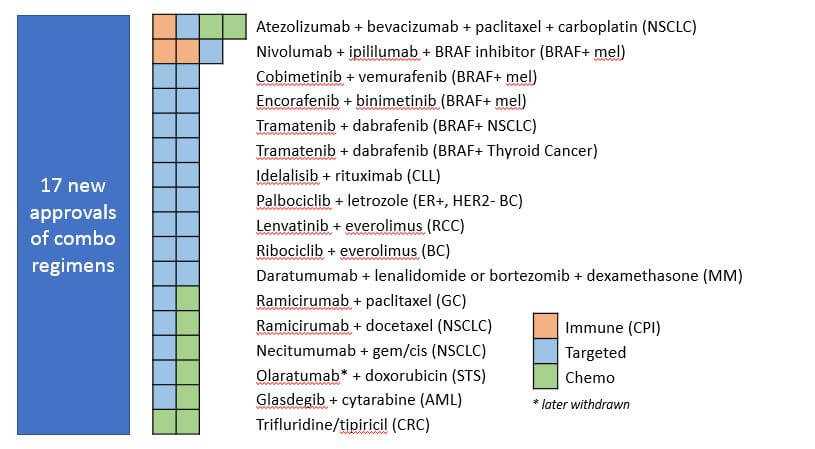 This signifies a general trend towards complementary or overlapping combination treatments designed to boost efficacy and / or preemptively target known tumor resistance pathways. For example, the PD1/CTLA4 doublets work in a complementary fashion by targeting two independent mechanisms of immune suppression, enabling much greater boosting of immune activity overall.
This signifies a general trend towards complementary or overlapping combination treatments designed to boost efficacy and / or preemptively target known tumor resistance pathways. For example, the PD1/CTLA4 doublets work in a complementary fashion by targeting two independent mechanisms of immune suppression, enabling much greater boosting of immune activity overall.
There have also been major advances in targeting known resistance pathways via combinations. For example, the introduction of the MEK inhibitor class of drugs enabled a combination strategy in BRAF mutated tumors. These tumors are known to develop resistance to BRAF inhibitor drugs given alone by increasing activation of MEK. Targeting MEK at the same time as BRAF negates this mechanism of resistance. Another example is the combination of a CDK4/6 inhibitor (ribociclib) with a PI3K inhibitor (letrozole) in BC, which targets overlapping mechanisms of resistance to hormonal therapies in breast cancer.
In addition to regimens that combine multiple drugs at the same time, we’ve seen advances in sequencing regimens. A notable example is the approval of the PARP inhibitor rucaparib and the PDL1 checkpoint inhibitor durvalumab as adjuvant or maintenance therapies in BRCA+ ovarian cancer and stage III NSCLC, respectively. Importantly, both have been shown to significantly delay recurrence with an overall survival benefit.
We are also seeing researchers and clinicians explore sequencing of the checkpoint inhibitors with other standard of care regimens to see which ones might prime or sensitize tumors to subsequent therapies. For example, a recent study in glioblastoma demonstrated that using the PD1 checkpoint inhibitor pembrolizumab before surgery (i.e., neoadjuvant treatment) significantly increased both progression-free survival as well as overall survival. This is a noteworthy finding in this very difficult to treat disease.
Importantly, our deeper understanding of tumor biology in specific settings enables a semi-personalized approach of aiming regimens at tumor biology (but not necessarily requiring molecular testing to confirm the whole regimen). For example, many of these new regimens do not require molecular testing at all, and for some of the ones that do have a biomarker-based indication, only one of the components is tested for; e.g., there is no biomarker testing done for use of the MEK inhibitor along with BRAF inhibitor, or for the anti-CTLA4, anti-PD1 combo used in melanoma. These tumor biology-based regimens therefore reflect an important step along the way towards truly personalized regimens.
Trend 4 – Diagnostic-Driven, Adaptive Regimens: Precision Medicine
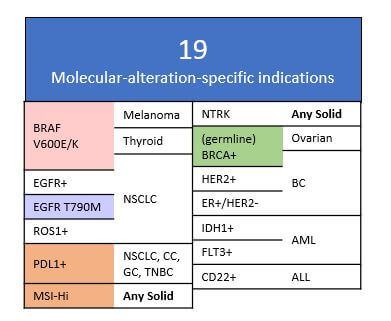
In addition to these trends of developing therapies towards novel targets as well as novel drug designs and regimens, we also see increasing emphasis on biomarker-directed usage of these drugs and regimens (e.g., indications defined by molecular alterations such as EGFR T790M mutation or high expression of PDL1). Of the ~90 labels for new drugs launched in the last 5 years, ~20% of them are for molecular alteration-defined indications.
We also observe a broadening of labels across additional tumor types based on the same specific molecular alteration, for example PARP inhibitors being first approved in germline BRCA+ ovarian tumors, followed by approval in germline BRCA+ TNBC. Recent data showing efficacy in germline BRCA+ pancreatic cancer suggests even broader potential. Even more striking has been the broad success of the PD(L)1 checkpoint inhibitors across both PDL1+ as well as all-comers indications in numerous tumor types.
Recently, two drugs achieved pan-tumor approvals based on molecular alterations alone, independent of tumor type:
- The checkpoint inhibitor pembrolizumab for any tumor with mismatch repair deficiency or microsatellite instability
- The NTRK inhibitor larotrectinib for any solid tumor with an NTRK activating fusion
Other efficacious targeted drugs may achieve pan-tumor clinical use and possibly even FDA approval in molecular alteration defined populations as well. For example, the PARP inhibitors in germline BRCA mutated tumors, or the BRAF/MEK inhibitor combos in any BRAF V600E mutated tumor. These mutation-directed approaches are already being used off-label fairly commonly and insurance companies have signaled willingness to cover based on early clinical data.
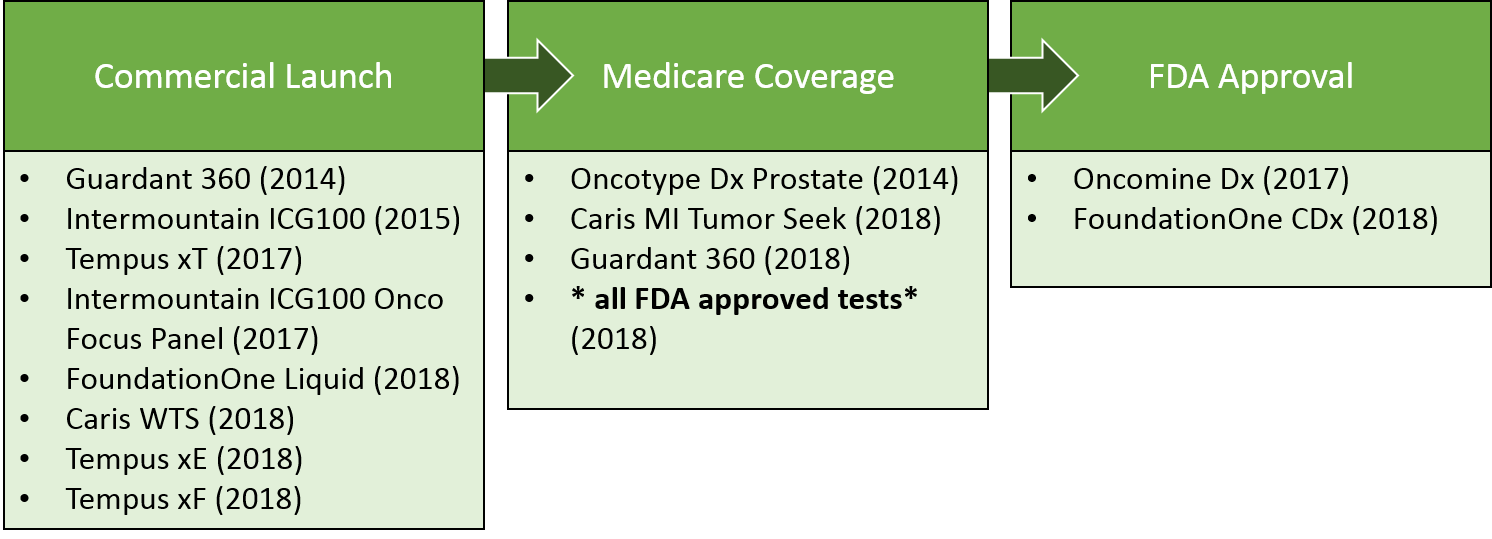
In addition to single gene companion diagnostics launched for many of these targeted therapies, there has been increasing use of broad panel tests. Over the past 5 years, these tests have achieved several key milestones towards commercialization. Notably, in 2018, Medicare announced coverage of all FDA approved molecular tests, ensuring easier patient access to Foundation One and other approved tests. That said, broad panel tests will continue to face challenges in demonstrating value, given the cost of the test vs. the relative rareness of identifying an actionable alteration for any given patient.
Ultimately, finely-tuned targeted therapies–along with broad diagnostic platforms to comprehensively identify the appropriate targets for each tumor–will continue to drive more personalized, adaptive regimens with improved outcomes.
We are already starting to see this in EGFR mutated NSCLC, where EGFR inhibitors work quite well for a while, but ultimately the tumor develops resistance and progresses. One common mechanism of resistance to EGFR inhibitors, the emergence of the EGFR T790M mutation, is targeted by the drug osimertinib. Because osimertinib also targets the general EGFR mutations, it is now the preferred first line therapy used to both address the EGFR driver mutation and also block resistance before it can take hold.
However, tumors typically develop a resistance to osimertinib in less than a year, often driven by the emergence of a separate mutation EGFR C797S. Intriguingly, the approved ALK inhibitor brigatinib can be combined with an anti-EGFR antibody to target this resistance mechanism in preclinical studies. This suggests a potential route to address this mechanism of resistance using an already approved drug. This type of adaptive treatment, along with the successful development of blood-based genomic panels enabling quick and easy identification of mutations, is a promising early example of truly personalized, adaptive regimens.
In addition to helping select effective treatments, broad panel tests can help identify treatments that are unlikely to work, saving patients from the risks of unnecessary treatments. For example, the Oncotype Dx breast test examines the expression of multiple disease-relevant genes and translates this biology into an assessment of how likely the tumor is to respond to chemotherapy.
This is an important question in early stage hormone positive breast cancer, where the question is frequently whether to do hormone treatment only, or hormone treatment plus chemo. For those tumors that are unlikely to respond to chemo, a patient and her doctor can leverage this test to more confidently skip the chemo (and its associated toxicity).
Another example is in colorectal cancer, where EGFR inhibitors can be quite effective unless the tumor has a KRAS or NRAS mutation which leads to EGFR resistance. In those cases, the EGFR approach should be avoided because it will not work.
We are also starting to see hints of how these tests may help people avoid treatment that is not just unlikely to work, but dangerous. For example, in a very small proportion of patients, the checkpoint inhibitor immunotherapies can trigger hyperprogression (rapid growth) of the cancer. Preliminary data has suggested that this outcome may be associated with amplification of the gene MDM2 and possibly EGFR mutations. So, when this finding shows up on a broad test like Foundation One or Caris, the informed treatment decision maker can implement a plan to monitor for hyperprogression if they do decide to use the checkpoint inhibitor drugs.
In part II of this series, we’ll take a look at the unmet needs that remain in oncology. We’ll also provide some thoughts regarding the future evolution of cancer therapies, given the trends we see today.