It’s no secret that change is a constant companion for those who work in the biopharmaceutical industry. That’s to be expected in any industry that thrives on innovation in the products it develops and the techniques it uses to develop them.
Over the past few years, one function has been transforming at a dramatically accelerated rate: Medical Affairs. This transformation has led to a significant boost in Medical Affairs’ strategic importance as a functional area. These changes now demand more of Medical Affairs team members, greatly expanding their responsibilities and pushing them to enhance their capabilities and skill sets.
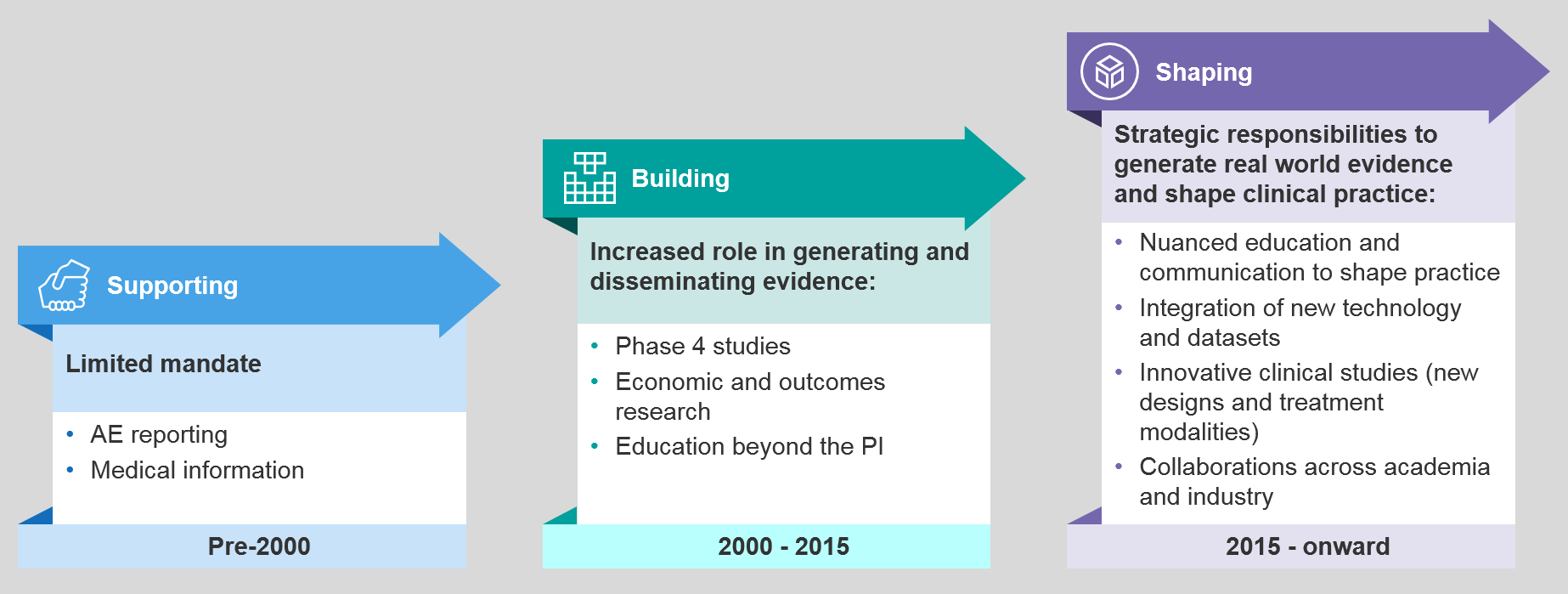
Drivers of Change
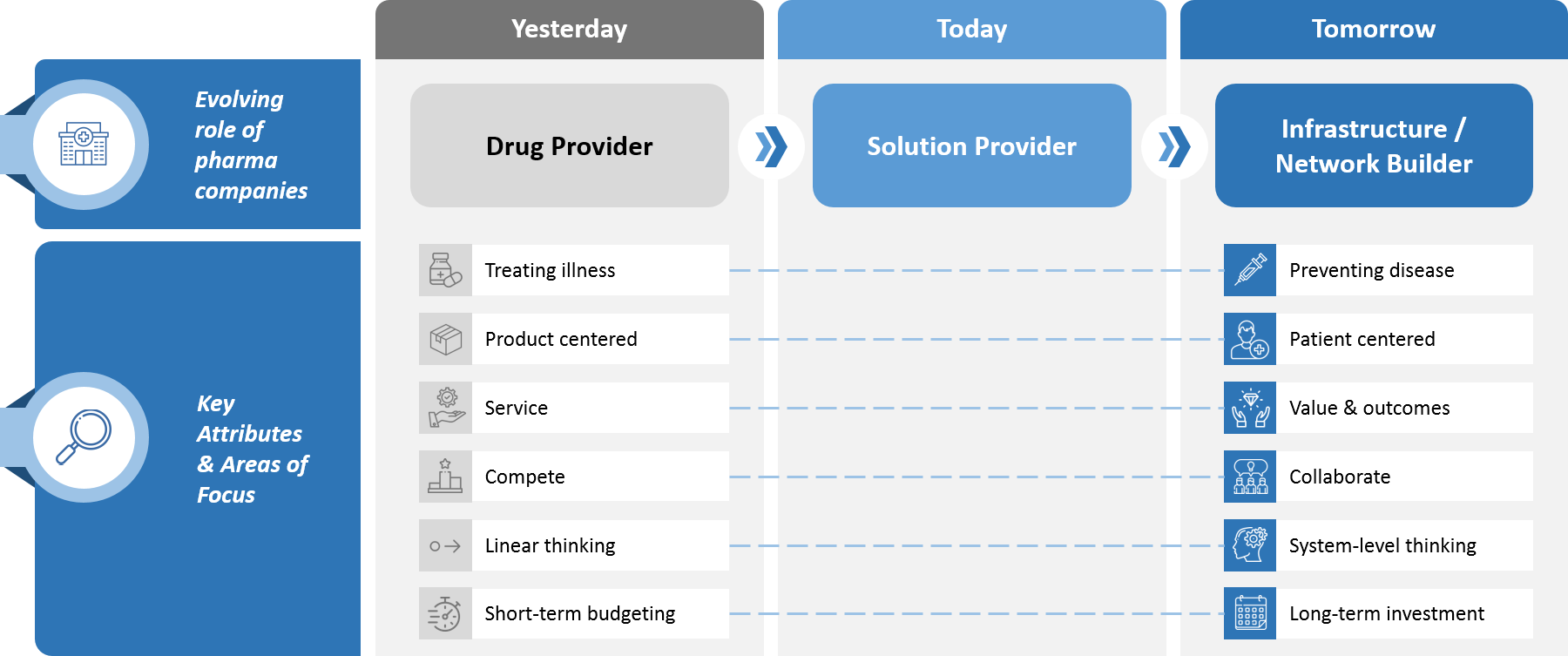
Medical Affairs’ transformation is not happening in a vacuum. Rather, it fits within a broader evolution that is taking place within the biopharma industry, as traditionally product-centered paradigms are supplanted by patient- and customer-centric solutions. (see Figure 2).
For Medical Affairs specifically, what’s behind all of this change? Based on our experience, we’ve identified several interrelated trends that are the primary drivers.
Trend 1: The Data Explosion
We are in the midst of an explosion in the availability of data. Rich, diverse, and complex data sources are now augmenting datasets from randomized, controlled clinical trials.
However, this growing volume of data could easily outpace the industry’s ability to derive insights from it. Increasingly, the challenge is how to optimally structure data, interpret it, and leverage it to make decisions. Because Medical Affairs is involved across the product development and commercialization cycle, this challenge can have implications for clinical study design, the collection and interpretation of real world evidence, medical education and communication, and more.
Medical Affairs teams—and biopharma companies in general—will need to partner with specialized organizations (and/or enhance their internal capabilities) to more effectively structure and analyze data at scale to inform strategic decision making. Innovative approaches using artificial intelligence and machine learning will likely factor into the industry’s solutions.
Trend 2: The Rise in Expedited Approvals
The increasing frequency of expedited approvals is also placing greater demands on Medical Affairs teams. New therapies can be brought to market earlier via emergency use authorizations, such as those for the COVID-19 vaccines, or other alternative accelerated pathways to market (an example is Project Orbis, which provides a framework for concurrent submission and review of oncology products among international partners). Additionally, the increasing use of adaptive trial designs or “phaseless” approaches to development can lead to faster market approvals, particularly for new cancer therapies.
Accelerated market approvals typically use smaller datasets, potentially leaving gaps that must be filled later. The result is a much greater demand for post-marketing studies and other data. In large part, it will fall to Medical Affairs to understand these gaps and help determine the best ways to fill them.
Trend 3: The Growing Volume and Diversity of Stakeholder Groups
Historically, Medical Affairs teams often focused on one primary stakeholder group: healthcare providers (HCPs). Today, they must engage with a much broader range of stakeholders including HCPs, patients, patient advocacy groups, societies and cooperative groups, academic institutions, payers, regulators, and others. Each stakeholder group has its own set of needs, motivations, expectations, and ways of communicating. This added complexity requires more sophisticated, tailored, and customer-friendly engagement approaches.
Implications for Medical Affairs
The trends mentioned above have a wide range of implications for Medical Affairs, reaching across its full spectrum of responsibilities.
- Insight Generation – Medical Affairs is evolving to become a critical nexus of cross-functional knowledge for internal partners and a trusted source for external stakeholders. As such, it will have an increasingly important role in gathering insights, communicating those insights throughout the organization, and leveraging them for decision-making. Moreover, it will be critical for Medical Affairs to do so rapidly and at scale to ensure that their pace of insight generation matches that of clinical practice needs.
- Evidence Generation – Obviously, Medical Affairs is not just a passive recipient of valuable data. It also plays a key role in generating it. Historically, data generation has been a siloed activity, with separate processes, priorities, and activities across different functions and regions. All that is changing. Now, there is a much greater need for comprehensive, integrated approaches to evidence planning and generation. Medical Affairs must lead the development of innovative approaches to generate robust evidence that is meaningful to regulators, payers, and the clinical community.
- Strategy Development – Industry trends are driving Medical Affairs to become an increasingly proactive strategic partner. Teams must provide strategic direction throughout the product lifecycle, informed by insights gathered from a broad range of customer, clinical, and other data sources. Solid strategic planning must include Medical Affairs teams as an integral member, and customer-based strategies must be aligned with other functional areas’ planning efforts.
- Stakeholder Engagement – As mentioned, Medical Affairs teams face a far more diverse universe of stakeholders than before. In addition, other teams within a biopharma company must also interact with many of the same stakeholder groups. This calls for compliant cross-functional coordination in planning and engagement. It’s critical to implement strategy effectively while preventing engagement efforts from becoming redundant, chaotic, or counter-productive.
- Communications Planning – While Medical Affairs must develop and communicate a compelling scientific narrative for each therapy it works with, it must also tailor that narrative appropriately to ensure its relevance to each stakeholder group. Compounding this challenge is the fact that the science is becoming increasingly complex (e.g., gene therapies, bi-specific antibodies, etc.). Medical Affairs must play a key role in making this complex science understandable—without losing accuracy or relevance—across all their audiences, both internal and external to the company. This will require Medical Affairs to use more complex and comprehensive approaches to communication planning.
Coming Next…
In upcoming articles, we will explore in greater detail the implications for Medical Affairs teams. Each subsequent article in this series will focus on a specific area of responsibility, such as Insight Generation, Evidence Generation, Strategy Development, Stakeholder Engagement, and Communications Planning. For each area, we will explore:
- Medical Affairs’ responsibilities in the area
- How those responsibilities are changing
- Case study examples of how Medical Affairs teams are adjusting to changes
- Guidance, insights, or best practices that medical affairs teams can use to navigate changes successfully
In the next installment, we dive into “Insight Generation.”









