Welcome to the second instalment of the Blue Matter monthly Health Technology Assessment (HTA) review, where we continue to monitor the European market access landscape and capture the pricing and reimbursement (P&R) outcomes in three top European markets: the UK, France, and Germany.
HTA Decisions in August 2023
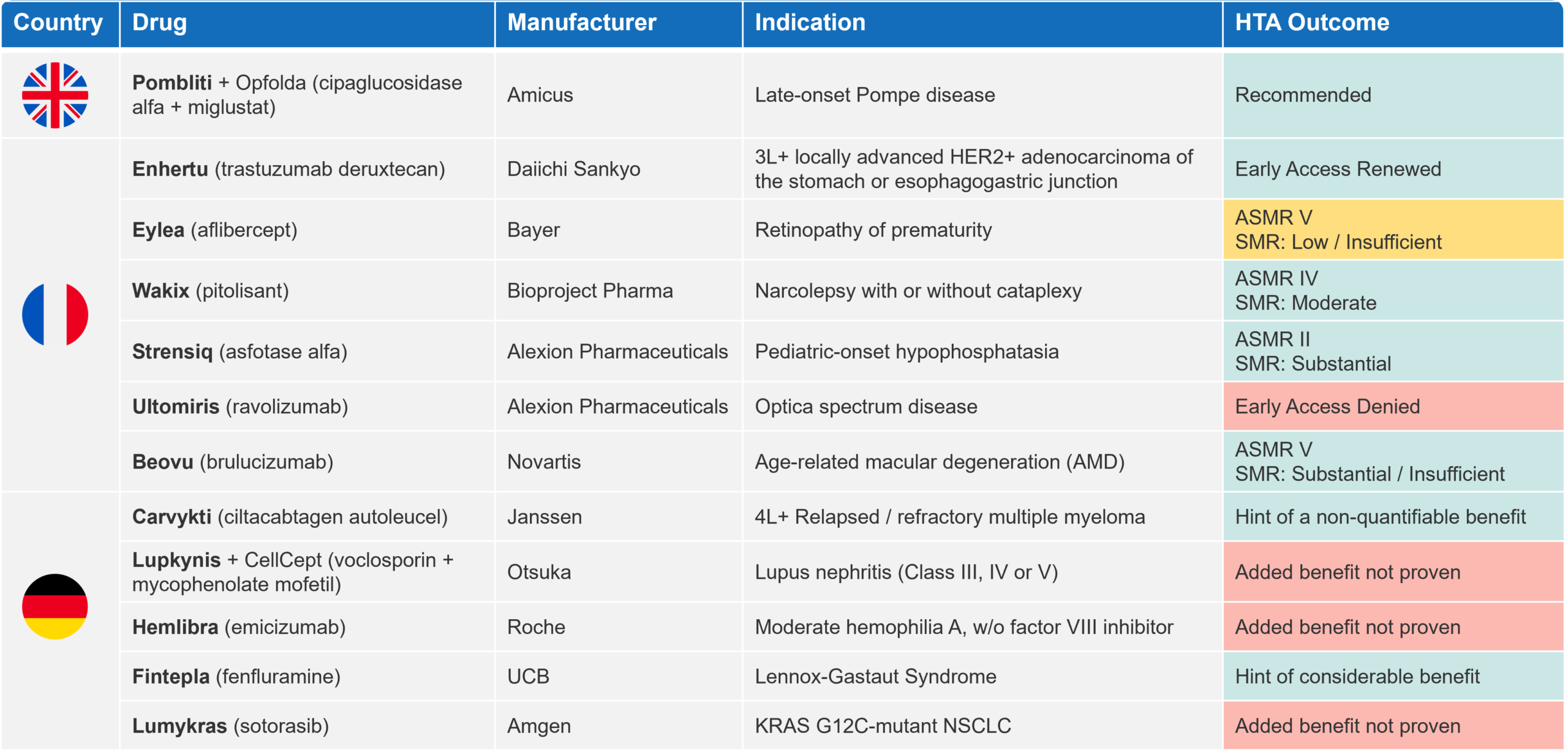
There continues to be high market access activity this summer in the pharmaceutical industry, with 21 P&R decisions made primarily in France and Germany (UK [1], France [10] and Germany [10]). Table 1 outlines the key HTA outcomes in oncology, rare diseases, and central nervous system (CNS) disorders, the latter two comprising the majority of P&R decisions in August.
Table 1 Key oncology, rare diseases and CNS HTA decisions in the UK, FR and DE in August 2023
In this article, we dive deeply into Strensiq® (asfotase alfa), which was able to maintain an ASMR II upon reassessment by the French National Health Authority (HAS). To provide insight into what is needed to justify and retain a high ASMR score, we will compare Strensiq with Elaprase® (idursulfase), which was unable to maintain its initial ASMR II at reassessment.
Achieving a “Successful” ASMR
Strensiq (asfotase alfa) – Initial Assessment by HAS
In Europe, Strensiq is the only approved treatment for patients with paediatric-onset hypophosphatasia (HPP)1, which is a rare inherited disease associated with inadequate bone mineralization2. Strensiq was initially assessed by HAS in 2016 and granted it ASMR II ‘Important’ and SMR ‘Substantial’.
To put this into perspective, HAS has granted a score of ASMR II to just ten products over the last five years. HAS’ decision was driven by the high disease burden and unmet need coupled with Strensiq’s efficacy demonstrated in four clinical studies across patient age groups. These studies were still ongoing at the time of initial assessment, but two were followed by an extension study for up to 7 years, which has not read out at this time of publication.
Considering all submitted efficacy and safety evidence, HAS acknowledged Strensiq’s clinical benefit in terms of radiological improvement of the wrist and knee joints (i.e., improved bone mineralisation). However, the Committee noted uncertainties associated with the long-term efficacy of Strensiq, the possibility of developing long-term anti-asfotase alfa or neutralizing antibodies, and the potential risk of extra-skeletal calcification.3 To address these uncertainties at reassessment, HAS requested evidence on mortality, morbidity, and quality of life (QoL) in French patients (treated and untreated). Alexion was able to leverage their existing International HPP Registry to address HAS’ request4.
Elaprase – Initial Assessment by HAS
Elaprase (idursulfase) is an orphan drug indicated for Hunter syndrome (mucopolysaccharidosis type II), a rare disease caused by enzyme deficiency, which is associated with cognitive impairments and early death, as well as a variety of musculoskeletal and cardiorespiratory complications.
The initial ASMR II was granted by HAS in 2007, driven by the high unmet need and notable benefit to public health that the therapy was expected to deliver. Although considered “weak” due to the small trial size and absence of mortality and morbidity data, the Phase III open-label trial met its primary composite endpoint. As part of the initial assessment, the Committee requested the establishment of a register of patients with Hunter syndrome in France to assess the impact of Elaprase on morbidity, mortality, QoL, and organisation of care over the long term5.
Reassessment Outcomes of Strensiq and Elaprase
Strensiq and Elaprase both achieved an ASMR II at their initial assessment. However, HAS noted uncertainties with respect to the long-term effectiveness of both therapies and their impact on mortality and/or morbidity. In both cases, HAS requested registry data on French patients, but was satisfied with the International Registries that both manufacturers had set up as part of their marketing authorisation process with the EMA. However, the two therapies achieved different outcomes upon re-evaluation:
At reassessment in 2023, Strensiq maintained its ASMR II by demonstrating sustained improvement in clinical benefit from ongoing trials, including evidence on long-term safety and efficacy up to seven years. In addition, data on the French population (n=86) from the International HPP Registry supported the efficacy and safety conclusions from the ongoing trials. The evidence presented also demonstrated improvement in respiratory function, physical parameters and QoL in patients with HPP. Finally, no treatment alternatives in HPP became available during the reassessment period, which maintained the unmet need addressed by Strensiq5.
At Elaprase’s reassessment in 2015, the manufacturer submitted long-term clinical data from the pivotal study (up to 3 years), a paediatric study and the French ancillary study based on the registry as per HAS’s request. Although Elaprase was still the only treatment option aside from haematopoietic stem cell transplant (HSCT), its ASMR score was reduced to IV due to insufficient evidence to indicate long-term effectiveness beyond the first year and a lack of mortality and/or morbidity data. During the 3-year follow up of its clinical trial, Elaprase did not demonstrate sustained (significant) improvement. In addition, the lack of a control group raised questions around the clinical relevance of the results6.
Insights from Strensiq & Elaprase on Achieving and Maintaining a “Successful” ASMR
There are many parallels for Strensiq and Elaprase in their initial evidence package. Although neither presented robust evidence on mortality and/or morbidity, both therapies were able to show clinical effect in the relevant populations at their initial assessment.
However, considering the trend among payers to be increasingly stringent and demanding for mortality and/or morbidity evidence, it could be assumed that the high unmet need and lack of alternative treatments in these rare diseases were the key drivers for the initial high ASMR scores.
At reassessment, the long-term evidence was enough to differentiate the two drugs. Strensiq presented evidence of sustained effect over nearly seven years, while Elaprase did not have sufficient evidence to justify maintenance of efficacy in the long-term. This also had pricing implications for the two therapies. As a result of lowering Elaprase’s ASMR score to IV, the list price went down by 7.5% in 2016, while Strensiq’s price remained the same upon its reassessment (discussions on confidential discounts are unknown).
While single-arm trials with short follow-up durations may be satisfactory for regulatory purposes and be sufficient for European payers to grant temporary reimbursement under viable commercial conditions, manufacturers will be required to demonstrate long-term efficacy if they want to maintain reimbursement at a commercially attractive price. Also, generating and presenting evidence on QoL can enhance the overall product value and improve a therapy’s ASMR score at re-assessment. In addition to this, payers have become more receptive to—and see high value in—real-world evidence (RWE), which proves effectiveness outside of randomized controlled trials.
Blue Matter Perspective and Recommendation
Achieving a positive reimbursement outcome in major European markets, such as France, has become challenging over the last decade. Stretched healthcare budgets, exacerbated during the COVID-19 pandemic, coupled with funding of high-priced products have led to increasingly stringent payer requirements for successful P&R. In France, what typically drives the product value in HTA assessment is the disease unmet need, trial design, product efficacy, safety, and QoL, and economic justification.
In conditions where there are alternative treatments, manufacturers need to demonstrate incremental benefit over the standard of care, ideally in a head-to-head (H2H) trial, if feasible. Failing to do so usually precludes them from achieving an ASMR IV or higher. While indirect treatment comparisons (ITCs) might be accepted, there must be a robust justification why a H2H trial was not performed, and the ITC should be performed in a way that meets HAS’ methodological requirements.
Many innovative products indicated in rare diseases achieve high ASMR scores at their initial assessment by HAS. This is largely driven by a high disease unmet need, which manufacturers can leverage to build their value story, plus demonstrating that there is a therapy that works in such a patient population despite the incompleteness of the evidence package. However, not all of them can sustain their ASMR rating at reassessment.
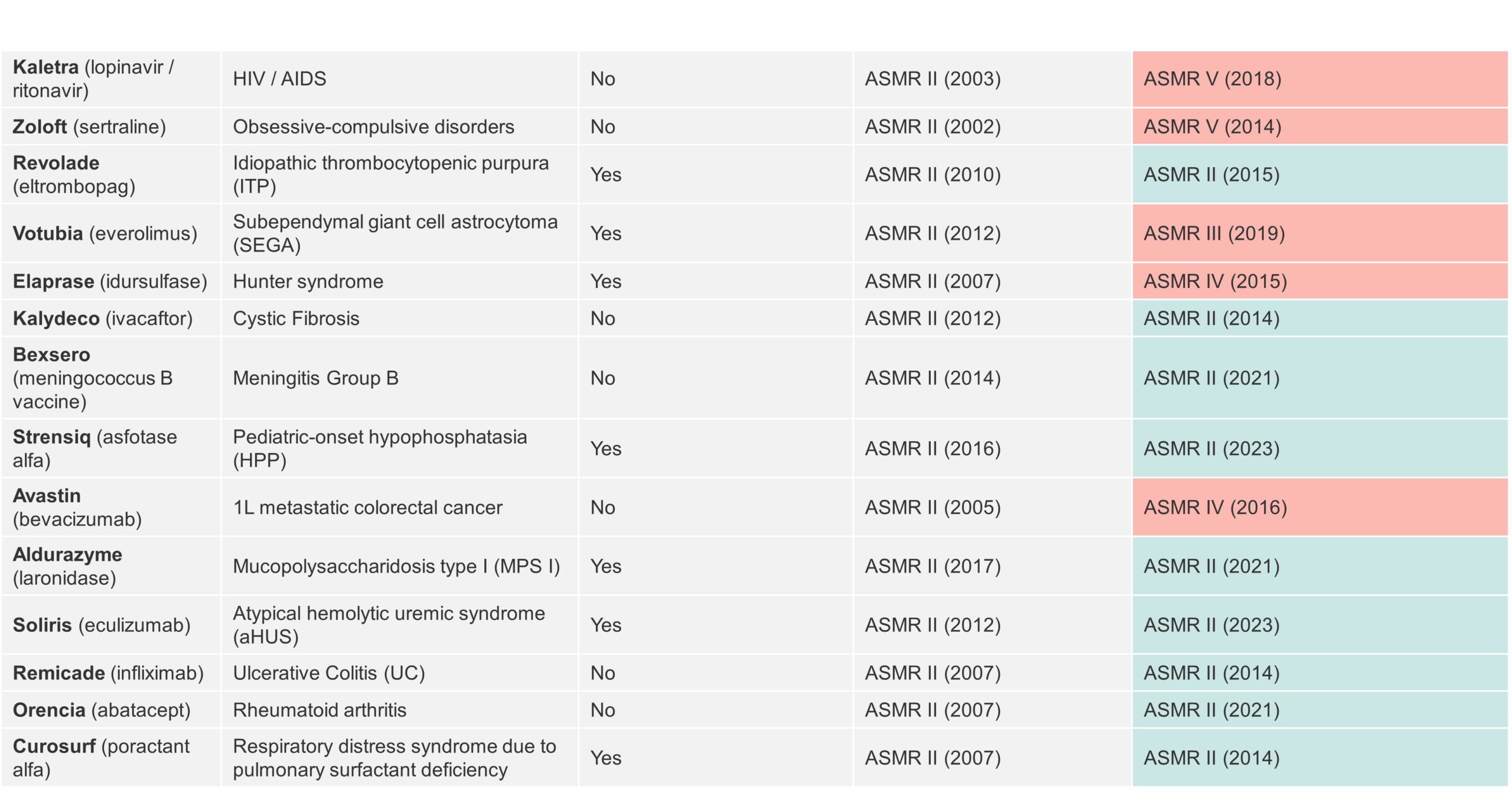
To gain a deeper perspective into the HAS reassessment process, we conducted an analysis on a wider sample of therapies that were able to achieve ASMR II at initial assessment. Our analysis captures 14 drugs, of which five (36%) had their ASMR score lowered at re-evaluation, while nine (64%) maintained their ASMR II score (Table 2).
Of the nine products that underwent a successful re-evaluation by HAS, five (56%) are indicated in rare diseases and four (45%) are approved in chronic conditions. Therefore, lack of alternative treatment options (8 of 9 diseases) rather than classification as a “rare disease” may play a major role in achieving a score of ASMR II. Of course, this must all be underpinned by evidence on sustained efficacy, including improvement in mortality and/or morbidity, which is the main value driver in HTA reassessments by HAS. Manufacturers will need to have a robust evidence generation strategy to satisfy HAS’s requirements and maintain their ASMR score at re-assessment, which is another hurdle that manufacturers need to overcome.
Table 2 Overview of treatments which have been reassessed by HAS upon achieving ASMR II (2002 – 2023)
Fortunately, RWE has established itself as a legitimate source of evidence for addressing any post-marketing authorisation uncertainties as it allows manufacturers to generate data on the utilisation of their products from real life. By doing so they can assess the long-term effectiveness of their products with respect to specific mortality and morbidity endpoints, which are often requested by payers around the globe.
Reduction of the ASMR rating naturally has implications on price in France. A further analysis on the price evolution of drugs shown in Table 2, which went from ASMR II to a lower rating, reveals that the price reduction upon reassessment is lower than one would anticipate: Elaprase’s list price went down by 7.5 % (ASMR II à IV), Votubia’s price dropped by 4.9% (ASMR II à III) and Kaletra’s price went down by 15.7% (ASMR II à V).
The impacts of confidential discounts are unknown. However, keeping a relatively high list price in France allows manufacturers to maintain viable prices across many other countries because France is one of the most internationally referenced markets as part of international reference pricing.
Conclusion
Conducting a comprehensive HTA landscape analysis and identifying potential data gaps early in the clinical development program can help manufacturers wisely plan their pivotal trial. This allows them to generate the necessary data by the time of HTA assessments and ensure the evidence is aligned with the needs of the local market. However, in addition to the clinical development programme, manufacturers should carefully plan their RWE strategy considering the increasingly important role that evidence from any post-registrational studies (e.g., registries, claims data etc.) plays in P&R negotiations with payers.
References:
- European Medicines Agency. Strensiq Summary of Product Characteristics (2023). https://www.ema.europa.eu/en/documents/product-information/strensiq-epar-product-information_en.pdf
- Tournis, S., Yavropoulou, M.P., Polyzos, S.A. and Doulgeraki, A., 2021. Hypophosphatasia. Journal of Clinical Medicine, 10(23), p.5676.
- HAS 2016. Strensiq (asfotase alfa), enzyme replacement therapy. Assessment. https://www.has-sante.fr/jcms/c_2621689/en/strensiq-asfotase-alfa-enzyme-replacement-therapy
- HAS 2023. Strensiq (asfotase alfa), enzyme replacement therapy. Reassessment. https://www.has-sante.fr/jcms/p_3455973/en/strensiq-asfotase-alfa-hypophosphatasie
- HAS 2007. Elaprase (idursulfase). Assessment. https://www.has-sante.fr/upload/docs/application/pdf/2010-04/elaprase_ct_4169.pdf
- HAS 2015. Elaprase (idursulfase). Reassessment. https://www.has-sante.fr/upload/docs/application/pdf/2016-04/elaprase_summary_ct13992.pdf